ALAS2 Antibody
-
货号:CSB-PA001560ESR2HU
-
规格:¥440
-
促销:
-
图片:
-
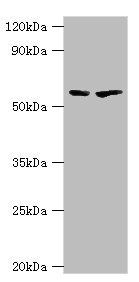
Western blot
All lanes: 5-aminolevulinate synthase, erythroid-specific, mitochondrial antibody at 7μg/ml
Lane 1: 293T whole cell lysate
Lane 2: Mouse brain tissue
Secondary
Goat polyclonal to rabbit IgG at 1/10000 dilution
Predicted band size: 65, 61, 50, 64 kDa
Observed band size: 65 kDa -
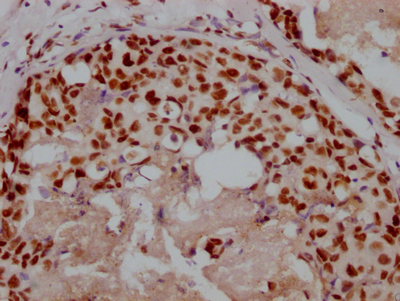
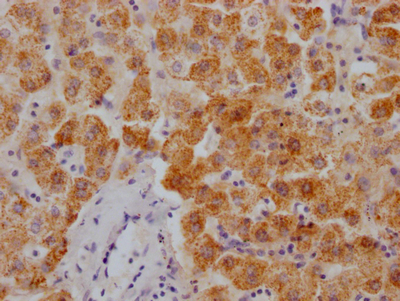
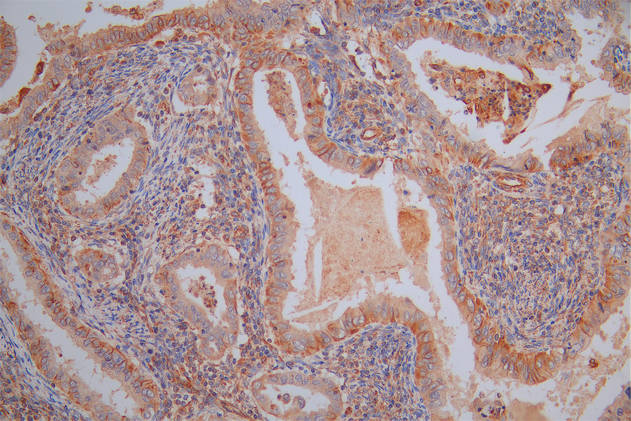
Immunohistochemistry of paraffin-embedded human tonsil tissue using CSB-PA001560ESR2HU at dilution of 1:100
-
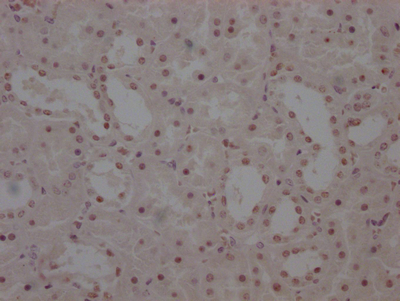
Immunohistochemistry of paraffin-embedded human esophagus tissue using CSB-PA001560ESR2HU at dilution of 1:100
-
-
其他:
产品详情
-
产品名称:Rabbit anti-Homo sapiens (Human) ALAS2 Polyclonal antibody
-
Uniprot No.:P22557
-
基因名:ALAS2
-
别名:5 @aminolevulinate synthase erythroid specific antibody; 5 aminolevulinate synthase 2 antibody; 5 aminolevulinate synthase 5 aminolevulinate synthase 2 antibody; 5 aminolevulinate synthase erythroid specific mitochondrial antibody; 5 aminolevulinic acid synthase 2 antibody; 5 aminolevulinic acid synthase antibody; 5-aminolevulinate synthase antibody; 5-aminolevulinic acid synthase 2 antibody; Alas 2 antibody; ALAS antibody; ALAS E antibody; ALAS; erythroid antibody; ALAS-E antibody; Alas2 antibody; ALASE antibody; Aminolevulinate delta synthase 2 antibody; Aminolevulinic acid synthase 2; erythroid antibody; ANH1 antibody; ASB antibody; Delta ALA synthase 2 antibody; Delta ALA synthetase antibody; Delta aminolevulinate synthase 2 antibody; Delta aminolevulinate synthase antibody; Delta-ALA synthase 2 antibody; Delta-aminolevulinate synthase 2 antibody; Erythroid specific ALAS antibody; erythroid-specific antibody; FLJ93603 antibody; HEM0_HUMAN antibody; mitochondrial antibody; OTTHUMP00000023388 antibody; OTTHUMP00000023389 antibody; OTTMUSP00000020679 antibody; RP23-338A17.1 antibody; SIDBA1 antibody; XLDPP antibody; XLEPP antibody; XLSA antibody
-
宿主:Rabbit
-
反应种属:Human, Mouse
-
免疫原:Recombinant Human 5-aminolevulinate synthase, erythroid-specific, mitochondrial protein (50-320AA)
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
-
克隆类型:Polyclonal
-
抗体亚型:IgG
-
纯化方式:Antigen Affinity Purified
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:PBS with 0.02% sodium azide, 50% glycerol, pH7.3.
-
产品提供形式:Liquid
-
应用范围:ELISA, WB, IHC
-
推荐稀释比:
Application Recommended Dilution WB 1:200-1:1000 IHC 1:20-1:200 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
基因功能参考文献:
- we report that the dynamics of ALAS2 active site loop is anti-correlated with the dynamics of the C-terminal tail and that this anti-correlation can represent a molecular basis for the functional and dynamic asymmetry of the ALAS2 homodimer. PMID: 29958424
- report confirms the considerable variability in manifestations among patients with ALAS2 or SLC25A38 mutations and draws attention to differences in the assessment and the monitoring of iron overload and its complications PMID: 28772256
- A novel ALAS2 missense mutation in exon 9 affects the enzymatic activity of ALAS2 by affecting its interaction with the cofactor pyridoxal 5'-phosphate in X-linked sideroblastic anemia. PMID: 28667034
- a case of X-linked sideroblastic anemia caused by a novel homozygous deletional mutation in exon 10 of ALAS2 gene is presented PMID: 28731922
- int-1-GATA site should be examined in patients with XLSA in clinical settings when no known mutation is found in ALAS2 exons. PMID: 28123038
- From pH jump experiments, comparable rates for the denaturation of the tertiary structure and PLP-microenvironment were discerned, indicating that the catalytic active site geometry strongly depends on the stable tertiary structural organization. Lastly, we demonstrate that partially folded ALAS tends to self-associate into higher oligomeric species at moderate GuHCl concentrations. PMID: 27751851
- data indicate that the X-linked protoporphyria variants possess enhanced ALAS activity and ALA dissociation rates, as well as distinct structural properties from those of wild-type hALAS PMID: 26300302
- In this article we add a novel mutation to the previously described 61 different ALAS2 mutations identified in X-linked sideroblastic anaemia patients. PMID: 24829177
- the primary deficiency in ferrochelatase leads to a secondary increase in ALAS2 expression. PMID: 25179834
- The ALAS2 Y365C mutation impairs pyridoxal 5'-phosphate binding to ALAS2, destabilizing the enzyme. X inactivation was not highly skewed in WBC from affected women. This X-linked dominant mutation perturbs erythropoiesis via cell-nonautonomous effects. PMID: 25705881
- the 130-base pair enhancer region located in the first intron of the ALAS2 gene should be examined in patients with congenital sideroblastic anemia in whom the gene responsible is unknown. PMID: 23935018
- 5 families with X-linked sideroblastic anemia had mutations in a GATA transcription factor binding site located in a transcriptional enhancer element in intron 1 of the ALAS2 gene. PMID: 24166784
- Loss-of-function FECH and gain-of-function erythroid-specific ALAS2 mutations causing erythropoietic protoporphyria and x-linked protoporphyria in North American patients reveal novel mutations and a high prevalence of X-linked protoporphyria. PMID: 23364466
- ALAS2 gain-of-function mutations increas the specific activity (DeltaAT, DeltaAGTG and p.Q548X) or stability (DeltaG) of the enzyme, thereby leading to the increased erythroid protoporphyrin accumulation causing X-linked protoporphyria. PMID: 23348515
- A large gain-of-function domain within the C-terminus of ALAS2 is associated with X-linked dominant protoporphyria. PMID: 23263862
- Late-onset photosensitivity was caused by ALAS2 mutation in a family with dominant protoporphyria. PMID: 23223129
- X-linked sideroblastic anemia due to carboxyl-terminal ALAS2 mutations that cause loss of binding to the beta-subunit of succinyl-CoA synthetase (SUCLA2). PMID: 22740690
- the C-terminal region of ALAS2 protein may function as an intrinsic modifier that suppresses catalytic activity and increases the degradation of its protein, each function of which is enhanced by the Met567Ile mutation and Val562Ala mutation, respectively PMID: 22269113
- Data suggest that ALAS2 gene mutations should be considered not only as causative of X-linked sideroblastic anemia (XLSA) and XLDPP but may also modulate gene function in other erythropoietic disorders. PMID: 21653323
- identification of five probands with sideroblastic anemia and ALAS2 R452S (due to SNP); all were African-American males; all presented with moderate anemia; the four adults presented with iron overload [a multi-case report from the United States] PMID: 21800356
- Thirteen different ALAS2 mutations were identified in 16 out of 29 probands with sideroblastic anemia. PMID: 21309041
- We found the previously published R452H and R452C ALAS2 mutations in 3 patients with X-linked sideroblastic anemia PMID: 21252495
- HIF1-mediated ALAS2 upregulation promotes erythropoiesis to satisfy the needs of an organism under hypoxic conditions. PMID: 21207956
- About 4% of unrelated EPP patients have X-linked dominant protoporphyria (MIM 300752) caused by gain-of-function mutations in the ALAS2 gene leading to an increased erythroid heme biosynthesis & protoporphyrin accumulation. Review. PMID: 20850938
- Seven ALAS2 mutations were detected in eight sporadic CSA cases, two being novel: V301A in a male patient and R517G in a female patient PMID: 19731322
- A novel mutation in exon 5 of the ALAS2 gene results in X-linked sideroblastic anemia. PMID: 12031592
- A C to G transversion at nucleotide -206 from the transcription start site was found in the proximal promoter region of ALAS2 in X-linked sideroblastic anemia. The region of the mutation may bind a novel and important erythroid regulatory element. PMID: 12663458
- the major splice isoform of ALAS2 is functional in vivo and could significantly contribute to erythroid heme biosynthesis and hemoglobin formation PMID: 14643893
- there is nucleotide variation at Msn and Alas2 on the X chromosome PMID: 15166166
- sequence identity of ALAS from Rhodobacter capsulatus and human eALAS is 49% PMID: 16121195
- ALAS2 mutations might contribute to more severe iron loading in persons with primary hemochromatosis. PMID: 16446107
- upon the NaBu stimulation, binding of Sp1 protein to ALAS2 promoter increased significantly, with concurrent increases in acetylation level of histone H3 and dimethylation level of H3-Lysine4 at ALAS2 promoter PMID: 18555711
- An impact of ALAD2 on blood lead levels or hemoglobin was not seen in Romanian women from a lead-contaminated region. PMID: 18569569
- gain-of-function mutations in ALAS2 cause a previously unrecognized form of porphyria, X-linked dominant protoporphyria, characterized biochemically by a high proportion of zinc-protoporphyrin in erythrocytes PMID: 18760763
- Multi-organ iron overload in an African-American man with ALAS2 R452S and SLC40A1 R561G. PMID: 19066423
- Hypoxia induces erythroid-specific 5-aminolevulinate synthase expression in human erythroid cells through transforming growth factor-beta signaling. PMID: 19187226
显示更多
收起更多
-
相关疾病:Anemia, sideroblastic, 1 (SIDBA1); Erythropoietic protoporphyria, X-linked dominant (XLDPT)
-
亚细胞定位:Mitochondrion matrix.
-
蛋白家族:Class-II pyridoxal-phosphate-dependent aminotransferase family
-
组织特异性:Erythroid specific.
-
数据库链接:
HGNC: 397
OMIM: 300751
KEGG: hsa:212
STRING: 9606.ENSP00000332369
UniGene: Hs.522666
Most popular with customers
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
-
-
-
-
-
-