CRYGS Antibody
-
货号:CSB-PA006025ESR1HU
-
规格:¥440
-
促销:
-
图片:
-
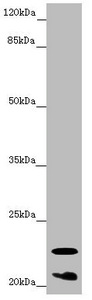
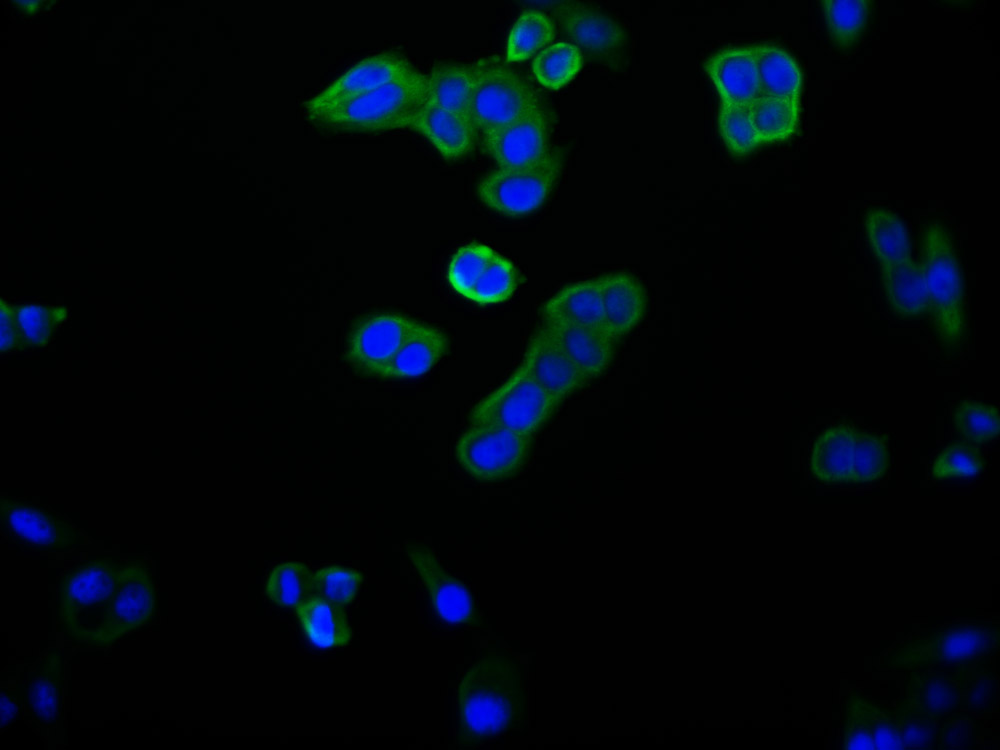
Western blot
All lanes: CRYGS antibody at 6.87 μg/ml + Mouse eye tissue
Secondary
Goat polyclonal to rabbit IgG at 1/10000 dilution
Predicted band size: 21 kDa
Observed band size: 21, 23 kDa -
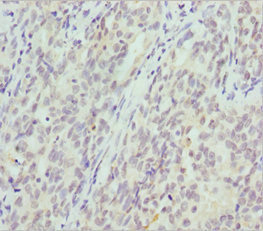
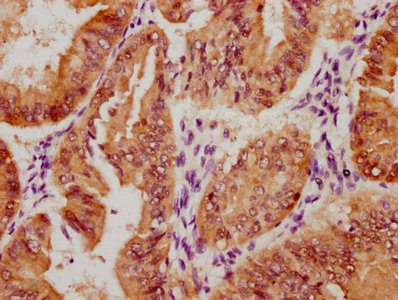
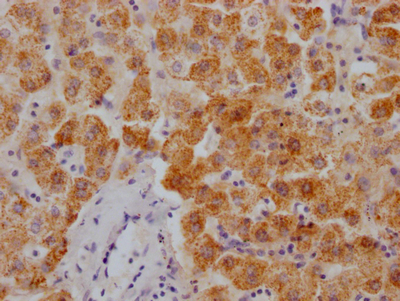
Immunohistochemistry of paraffin-embedded human lung cancer using CSB-PA006025ESR1HU at dilution of 1:100
-
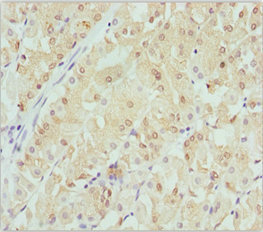
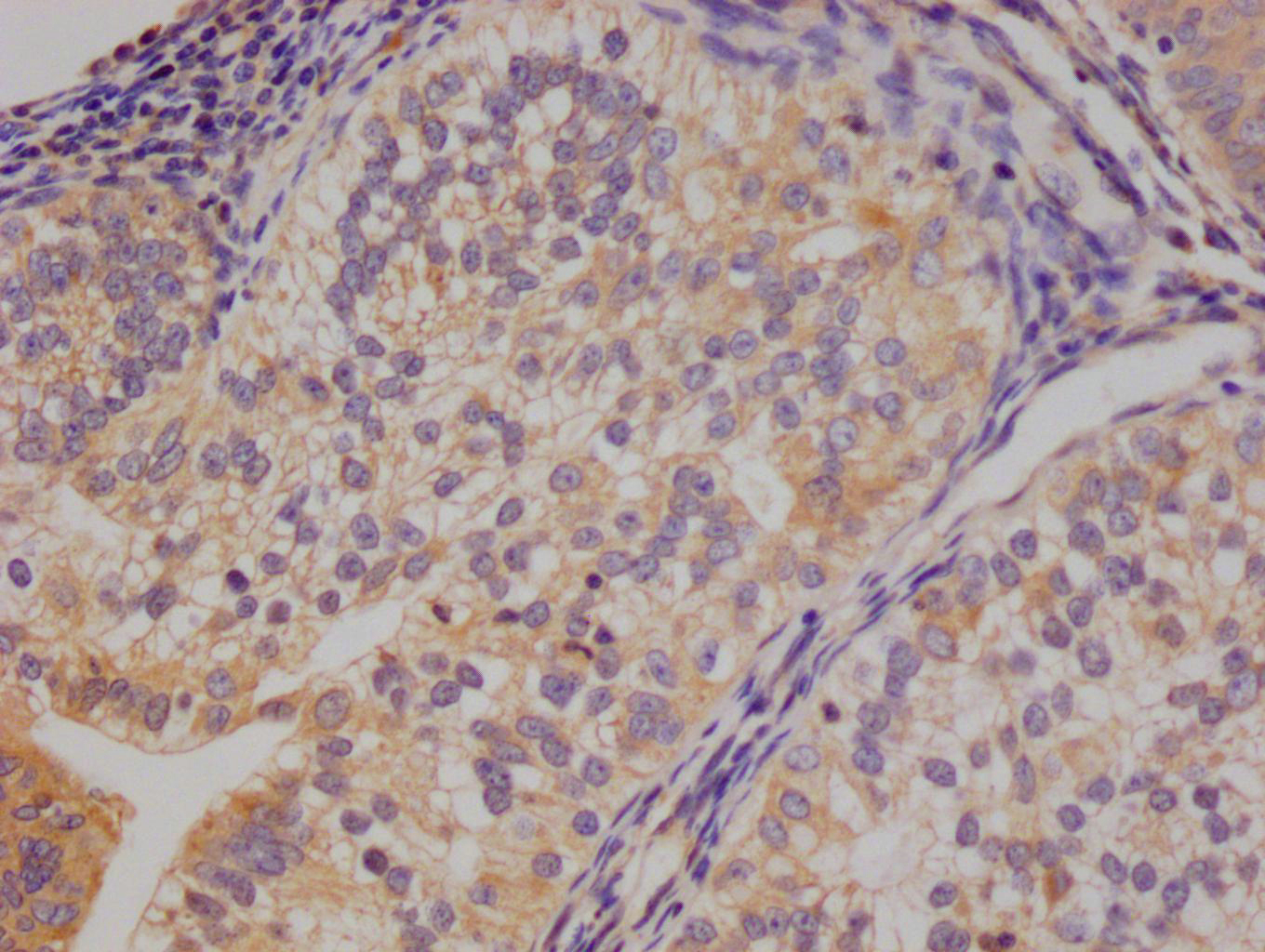
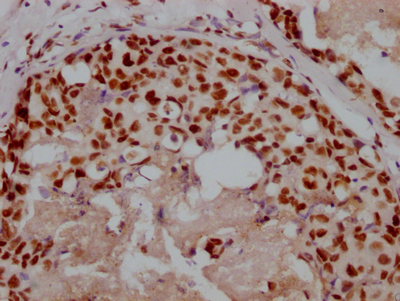
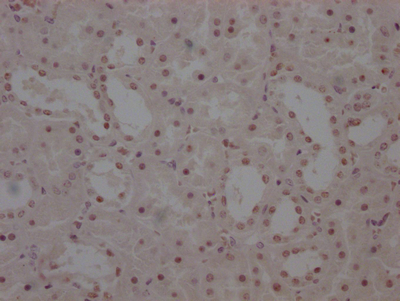
Immunohistochemistry of paraffin-embedded human gastric cancer using CSB-PA006025ESR1HU at dilution of 1:100
-
-
其他:
产品详情
-
产品名称:Rabbit anti-Homo sapiens (Human) CRYGS Polyclonal antibody
-
Uniprot No.:P22914
-
基因名:CRYGS
-
别名:AI327013 antibody; Beta-crystallin S antibody; CRBS_HUMAN antibody; CRYG8 antibody; crygs antibody; Crystallin; gamma 8 antibody; Crystallin; gamma polypeptide 8 antibody; Crystallin; gamma S antibody; CTRCT20 antibody; Gamma crystallin S antibody; Gamma S crystallin antibody; Gamma-crystallin S antibody; Gamma-S-crystallin antibody; Opacity due to poor secondary fiber cell junction; recessive nuclear cataract antibody; Opj antibody; rncat antibody
-
宿主:Rabbit
-
反应种属:Human, Mouse
-
免疫原:Recombinant Human Beta-crystallin S protein (1-178AA)
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
-
克隆类型:Polyclonal
-
抗体亚型:IgG
-
纯化方式:Antigen Affinity Purified
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:PBS with 0.02% sodium azide, 50% glycerol, pH7.3.
-
产品提供形式:Liquid
-
应用范围:ELISA, WB, IHC
-
推荐稀释比:
Application Recommended Dilution WB 1:1000-1:5000 IHC 1:20-1:200 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Crystallins are the dominant structural components of the vertebrate eye lens.
-
基因功能参考文献:
- aberrant modifications in gammaS-crystallin structure might contribute to the lower stability and higher aggregatory potency of the mutated protein, which subsequently resulted in cataracts in the patients PMID: 29857103
- The Tyr67Asn substitution was predicted to decrease the local hydrophobicity and affect the three-dimensional structure of gammaS-crystallin, and resulted in a portion of mutant protein translocation from the cytoplasm to cell membrane. This observations expand the mutation spectrum of CRYGS and provide further evidence for the genetic basis and molecular mechanism of congenital cataract. PMID: 29964096
- Cataract-related G18V point mutation affects CRYGS stability and hydration. PMID: 27052457
- novel mutation (G57W) in CRYGS in this Chinese family is associated with autosomal dominant pulverulent cataract. PMID: 24328668
- The data suggest that enhanced attractive protein-protein interactions, arising from the deamidation of HGS, promote protein aggregation, thereby leading to increased light scattering and opacity over time. PMID: 26158710
- The effects of the V41M mutation on the structural changes of gamma S-crystallin were studied. PMID: 24287181
- The cataract-associated mutant D26G of human gammaS-crystallin is remarkably close to the wild type molecule in structural features, with only a microenvironmental change in the packing around the mutation site. PMID: 23761725
- replacement of valine in position 42 by the longer and bulkier methionine in human gammaS-crystallin perturbs the compact beta-sheet core packing topology in the N-terminal domain of the molecule PMID: 23284690
- age-dependent cleavage of gammaS-crystallin generates a peptide that binds to cell membranes PMID: 22995907
- The degree of deamidation for Gln92 and Gln170 was found to increase from birth to teen-age years and then to remain constant for four decades. PMID: 22593035
- Molecular dynamics (MD) simulations, circular dichroism (CD), and dynamic light scattering (DLS) measurements were used to investigate the aggregation propensity of the eye-lens protein gammaS-crystallin. PMID: 21244846
- Partially folded aggregation intermediates of human gammaD-, gammaC-, and gammaS-crystallin are recognized and bound by human alphaB-crystallin. PMID: 20621668
- Deamidation in cataractous lenses is influenced by surface exposure. PMID: 12093281
- A lens gamma S-crystallin has been identified with an in vivo modification, S-methylation of cysteine residues, that may block intermolecular disulfide bondng and serve as a form of protection against cataract. PMID: 12475213
- when glutathione becomes bound to gammaS-crystallin, it causes it to bind in turn to the beta-crystallin polypeptides to form a dimer PMID: 14763903
- report a novel missense mutation, p.V42M, in CRYGS associated with bilateral congenital cataract in a family of Indian origin PMID: 19262743
- Fast charge transfer quenching is an evolved property of the gamma S-crystallin fold, probably protecting it from ultraviolet-induced photodamage. PMID: 19358562
- Results confirm the high stability of wild-type HgammaS-crystallin and demonstrates that the G18V mutation destabilizes the protein toward heat and GuHCl-induced unfolding. PMID: 19558189
显示更多
收起更多
-
相关疾病:Cataract 20, multiple types (CTRCT20)
-
蛋白家族:Beta/gamma-crystallin family
-
数据库链接:
HGNC: 2417
OMIM: 116100
KEGG: hsa:1427
STRING: 9606.ENSP00000312099
UniGene: Hs.376209
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-