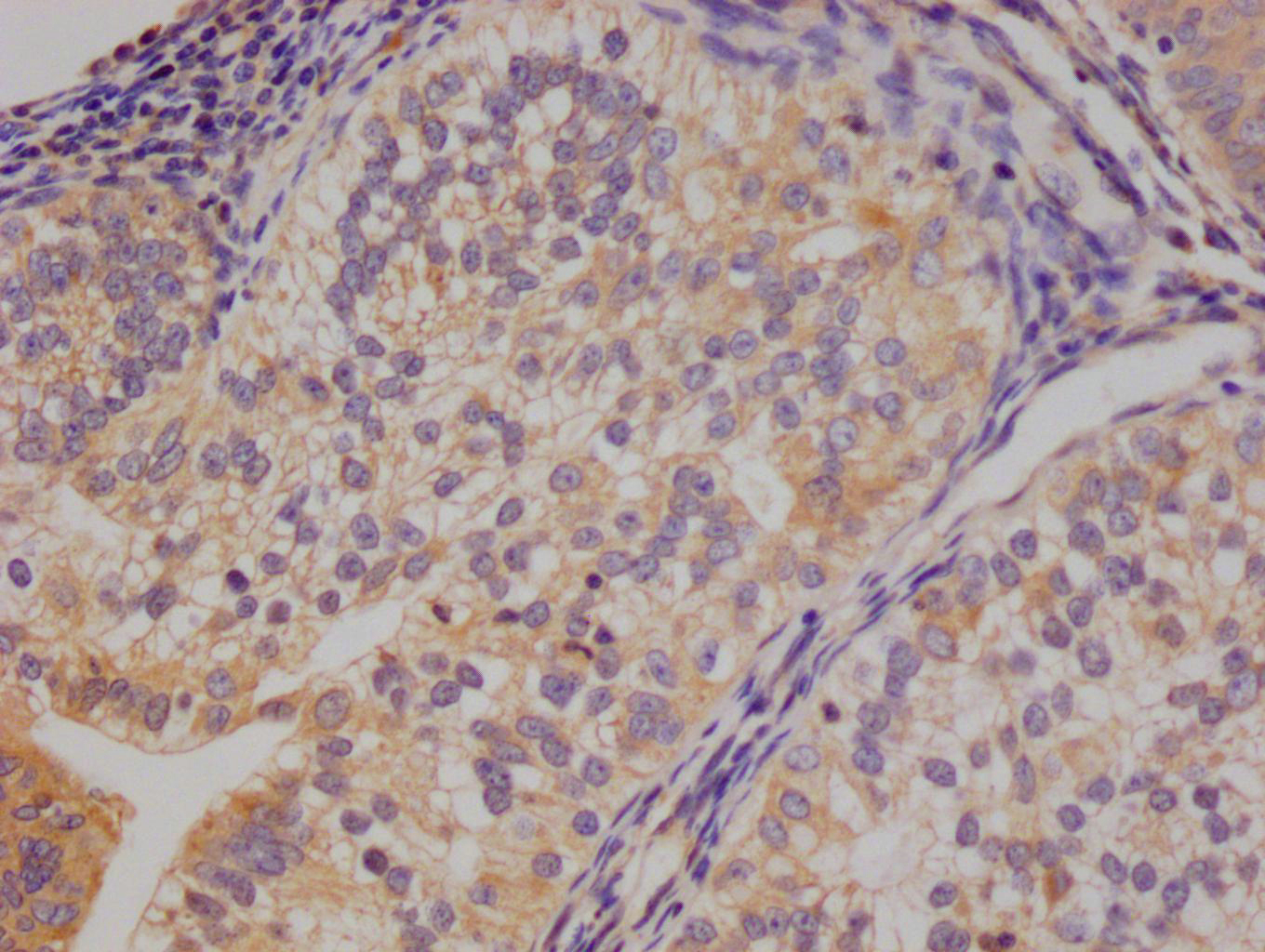
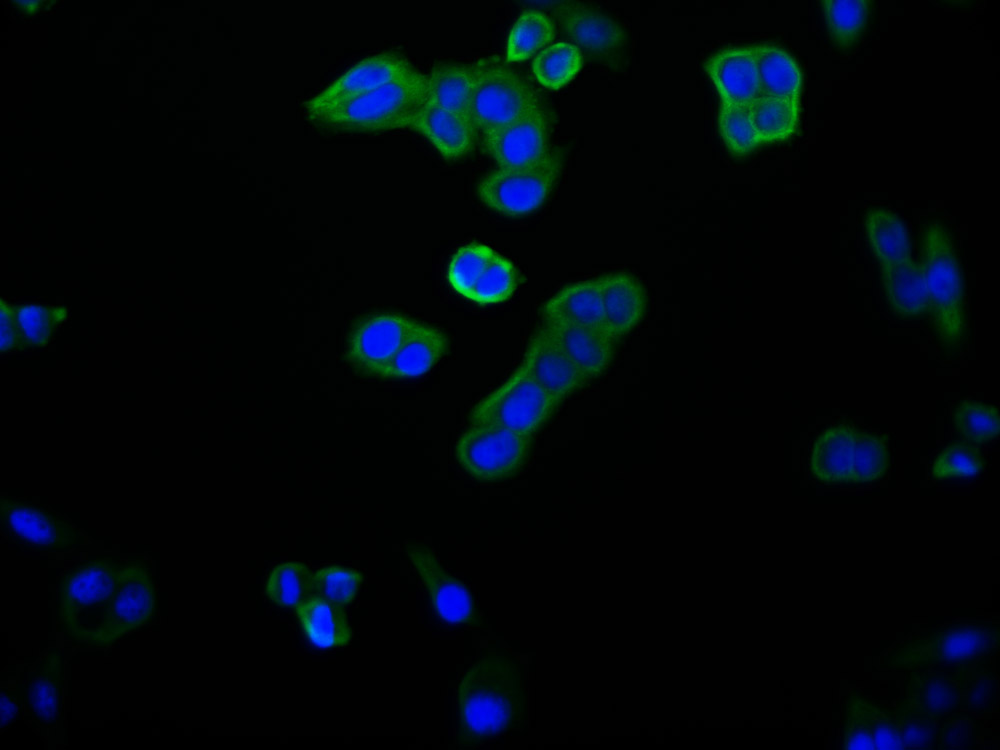
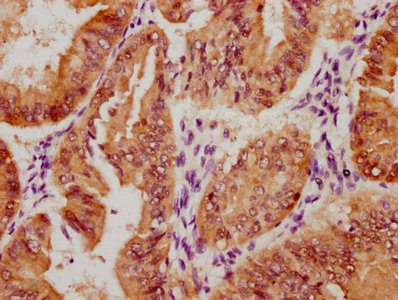
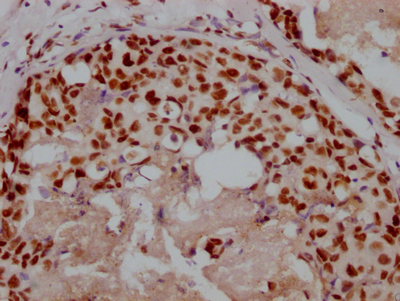
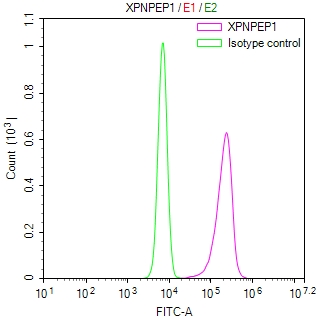
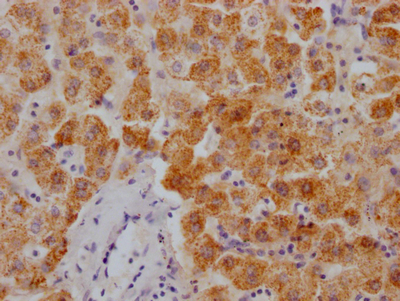
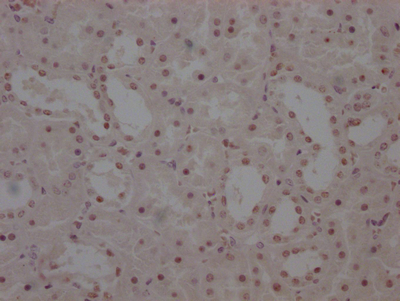
PRNP Antibody
-
货号:CSB-PA018739GA01HU
-
规格:¥3,900
-
其他:
产品详情
-
Uniprot No.:P04156
-
基因名:PRNP
-
别名:Alternative prion protein, major prion protein antibody; AltPrP antibody; ASCR antibody; CD230 antibody; CD230 antigen antibody; CJD antibody; GSS antibody; KURU antibody; Major prion protein antibody; p27 30 antibody; PRIO_HUMAN antibody; Prion protein antibody; Prion related protein antibody; PRIP antibody; PRNP antibody; PrP antibody; PrP27 30 antibody; PrP27-30 antibody; PrP33-35C antibody; PrPC antibody; PrPSc antibody; Sinc antibody
-
宿主:Rabbit
-
反应种属:Human,Mouse,Rat
-
免疫原:Human PRNP
-
免疫原种属:Homo sapiens (Human)
-
抗体亚型:IgG
-
纯化方式:Antigen Affinity Purified
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:PBS with 0.1% Sodium Azide, 50% Glycerol, pH 7.3. -20°C, Avoid freeze / thaw cycles.
-
产品提供形式:Liquid
-
应用范围:ELISA,WB,IHC
-
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Its primary physiological function is unclear. May play a role in neuronal development and synaptic plasticity. May be required for neuronal myelin sheath maintenance. May promote myelin homeostasis through acting as an agonist for ADGRG6 receptor. May play a role in iron uptake and iron homeostasis. Soluble oligomers are toxic to cultured neuroblastoma cells and induce apoptosis (in vitro). Association with GPC1 (via its heparan sulfate chains) targets PRNP to lipid rafts. Also provides Cu(2+) or ZN(2+) for the ascorbate-mediated GPC1 deaminase degradation of its heparan sulfate side chains.
-
基因功能参考文献:
- interaction site of peptide aptamer 8 in PrP and modeled the complex in silico to design targeted mutations in PA8 which presumably enhance binding properties. PMID: 29460268
- these data suggest that PrP protects cells against premature senescence induced by copper. PMID: 28800967
- These findings divulge a new cellular response that is activated upon CsA treatment to secrete misfolded PrP species from the cell and may underlie the spreading of toxic prions among cells and across tissues. PMID: 29127190
- Luman, a ubiquitous, non-canonical unfolded protein response (UPR), is identified as a novel regulator of endoplasmic reticulum stress-induced PRNP expression. PMID: 28205568
- These findings reveal that PrP enhances the responses to TNF-alpha, promoting proinflammatory cytokine production, which may contribute to inflammation and tumorigenesis. PMID: 28900035
- Bank vole asparagine and glutamine residues enable prion conversion of human and rabbit PrPC. PMID: 28931606
- Unlike the western populations, the diverse phenotypical presentations of D178N mutants of PRNP were not simply determined by the 129 genotypes in Chinese PMID: 29569252
- The octarepeat region within the PrP peptide markedly influences the effects of redox on the biochemical phenotypes of PrP, thus highlighting the importance of the number of octarepeats in the biological functions of PrP. PMID: 29393338
- Genetic prion diseases (gPrDs) are caused by autosomal-dominant mutations in the prion protein gene (PRNP). PMID: 29478593
- All these data suggest the possibility that hypoxiamediated PrPC serves an important role in angiogenesis. Therefore, the present review summarizes the characteristics of PrPC, which is produced by HIF1alpha in hypoxia, as it relates to angiogenesis PMID: 28901450
- This study is the first to demonstrate that tauroursodeoxycholic acid protects MSCs against ER stress via Akt-dependent PrP(C) and Akt-MnSOD pathway. PMID: 28004805
- The stabilization mechanism of specific binding compounds can be summarized as stabilizing both the flexible C-terminal of alpha2 and the hydrophobic core, or only the hydrophobic core, or the overall structure of PrP(C) by high binding affinity. N159 and Q160 play an major role in the specific binding of the studied compounds, all of which interact similarly with L130, P158, N159, Q160,H187, T190, T191. PMID: 28795797
- disease-associated mutations provide valuable insights into possible key structural determinants underlying misfolding of PrPC (review) PMID: 28838676
- This study reports a novel p.S17G mutation in a clinically diagnosed LOAD patient, suggesting that the PRNP mutation is present in Chinese Alzheimer's disease patients, whereas, M129V polymorphism is not a risk factor for Alzheimer's disease or frontotemporal dementia in the Chinese Han population. PMID: 27910931
- Those data indicate that the overexpression of PLK3-mediated degradation of abnormal PrP is largely dependent on chaperone-mediated autophagy pathway. PMID: 27344333
- biochemical characteristics of valine-to-isoleucine substitution at codon 180 (V180I) in PRNP gene from autopsied brains of patients with genetic Creutzfeldt-Jakob disease; findings indicate abnormal prion proteins in the neocortex are associated with toxicity resulting in severe spongiosis and that V180I is not a polymorphism, but is an authentic pathogenic mutation associated with specific biochemical characteristics PMID: 29382530
- mechanism of the unfolding of the human prion protein PMID: 28030950
- Here, we provide an overview of the increasingly multifaceted picture of prion protein proteolysis and shed light on physiological and pathological roles associated with these cleavages. PMID: 28693923
- the coordination bonds between the Methionine-Lysine-Histidine (Ac-MKH-NHMe) tripeptide model associated with the fifth metal binding site, which triggers the beta-sheet formation of human prion protein and the divalent metal cations such as Mn(2+), Cu(2+) and Zn(2+) were studied. PMID: 27611644
- Importantly, flies expressing human PrP showing a robust eye phenotype will allow performing genetic screens to uncover novel mechanisms mediating PrP neurotoxicity. PMID: 28415023
- Our results indicated, we found that PrP gene had an IRES-dependent translation initiation mechanism and we successfully identified the IRESs inside of the prion protein gene. PMID: 29107182
- the kinetics of prion replication occur in a prion protein codon 129 genotype-dependent manner, reflecting the genotype-dependent susceptibility to clinical variant Creutzfeldt-Jakob disease found in patients. PMID: 29141869
- the modulation of HOP-PrP(C) engagement or the decrease of PrP(C) and HOP expression may represent a potential therapeutic intervention in glioblastoma. PMID: 28412969
- a strong overexpression of PrP(C) is observed in human Merlin-deficient mesothelioma cell line TRA and in human Merlin-deficient meningiomas. PrP(C) contributes to increased proliferation, cell-matrix adhesion and survival in schwannoma cells acting via 37/67 kDa non-integrin laminin receptor (LR/37/67 kDa) PMID: 28692055
- Homozygous state of position 129 in the PRNP is not a risk factor for MSA. No other variants in the PRNP gene were associated with increased risk for MSA PMID: 27793473
- Transgenic Creutzfeldt-Jakob disease (CJD) mice, expressing the mouse PrP (moPrP) homolog of human PrP D178N/V129 (moPrP D177N/V128), closely reproduce essential features of CJD. The mutant PrPs expressed in these mice are misfolded but unable to self-replicate. They accumulate in different compartments of the neuronal secretory pathway, impairing the membrane delivery of ion channels essential for neuronal function. PMID: 26864450
- Identified are several nuclear PrP(c) partners, which comprise gamma-catenin, one of its desmosomal partners, beta-catenin and TCF7L2, the main effectors of the canonical Wnt pathway, and YAP, one effector of the Hippo pathway. PMID: 27216988
- Rare mutation in PRNP leading to an exchange of amino acid from glutamic acid (E) to alanine (A) at codon 196 (E196A) is associated with Creutzfeldt-Jacob disease. PMID: 27310471
- This article discusses a framework for investigating the extended hypothesis that PrPC may be involved in major depression associated with neurodegenerative conditions, with focus on the Transmissible Spongiform Encephalopathies (TSEs, or Prion Diseases) and Alzheimer's Disease (AlzD). PMID: 27057694
- Expert commentary: Computational approaches provide novel insights into prion-like protein functions, their regulation and their role in disease. PMID: 28271922
- We suggest that reduction of PFN-1 expression by elevated levels of PrP(c) may contribute to protective effects PrP(c)-overexpressing SH-SY5Y cells confer against STS-induced apoptosis PMID: 28102851
- findings show that sPrPc is involved in the processes of HIV neuropathogenesis and contributes to inflammation and neuronal damage PMID: 28533442
- Copper(II) interaction with the Human Prion 103-112 fragment and its mutants has been studied with various techniques. The studied human prion fragment contains both histidine and methionine residues, while methionine residues are systematically replaced or displaced in the studied mutants PMID: 28260678
- these data indicate that the disruption of the PrP(C)-HOP complex could be a potential therapeutic target for modulating the migratory and invasive cellular properties that lead to metastatic Colorectal cancer (CRC). PMID: 27112151
- computational approach to elucidate in details the aggregation propensity of PrP protein systems including wild type, wild type treated at different [Ca2+] or E200K mutant; models for the self-assembly of either the E200K mutated or Ca2+-bound PrPC were sketched and discussed PMID: 27959938
- The results have unraveled a novel molecular pathway driven by interactions between prion protein (PrP) and Notch1 in the progression of pancreatic ductal adenocarcinoma (PDAC), supporting a critical tumor-promoting role of Notch1 in PrP-expressing PDAC tumors. PMID: 27639164
- The present findings unveil particular neuropathological and neuroinflammatory profiles in Fatal familial insomnia(FFI) and novel characteristics of natural prion protein in FFI, altered PrPres and Scrapie PrP (abnormal and pathogenic PrP) patterns and region-dependent putative capacity of PrP seeding PMID: 27056979
- the data indicate a four-rung beta-solenoid structure as a key feature for the architecture of infectious mammalian prions. PMID: 27606840
- Distinctive properties of plaque-type dura mater graft-associated Creutzfeldt-Jakob disease PrPSc proteins in cell-protein misfolding cyclic amplification. PMID: 26878132
- Molecular insights obtained through MD (molecular dynamics) simulations suggested that each bispidine-based peptidomimetic differently engages a conserved Tyr 169 residue at the alpha2-beta2 loop of HuPrP and affects the stability of alpha2 and alpha3 helices. PMID: 27803245
- These data identify a network of proteins implicated in PrP(C) trafficking and demonstrate the power of this assay for identifying modulators of PrP(C) trafficking. PMID: 28341739
- Active compounds do not alter total or cell-surface levels of PrP(C), and do not bind to recombinant PrP in surface plasmon resonance experiments, although at high concentrations they inhibit PrP(Sc)-seeded conversion of recombinant PrP to a misfolded state in an in vitro reaction (RT-QuIC). PMID: 27803163
- data provided molecular details about the interaction between HuPrP and the NCAM fibronectin domain, and revealed a new role of PrP(C) N terminus as a dynamic and functional element responsible for protein-protein interaction. PMID: 27535221
- This work sheds light on the amyloid core structures underlying prion strains and how I138M, I139M, and S143N affect prion protein aggregation kinetics. PMID: 27576687
- Data suggest second and third helices (H2 and H3) of C-terminal region of prion protein serve as Achilles heels of prion protein stability; separation of B1-H1-B2 and H2-H3 domains seems to play a key role, as well. (H1, H2, H3 denote the 3 alpha-helices; B1, B1 denote the 2 beta-sheets. Studies involved molecular dynamic simulations using nuclear magnetic resonance data obtained for N-terminal and C-terminal domains.) PMID: 28102071
- prion protein-derived cell-penetrating peptide cytotoxicity is modulated by pH but independent of amyloid formation PMID: 27818203
- effect of familial Creutzfeld-Jacob disease prion genes on prion protein conformation and secondary structure PMID: 27107654
- The two cases reported here of sporadic Creutzfeldt-Jakob disease belonged to the same family and carried the most common point mutation of the PRNP gene observed in Italy. PMID: 26268049
- The protonation state of histidine 111 regulates the aggregation of the evolutionary most conserved region of the human prion protein. PMID: 27184108
- This study demonstrated that Prion Protein-Hemin Interaction Upregulates Hemoglobin Synthesis. PMID: 26836195
显示更多
收起更多
-
相关疾病:Creutzfeldt-Jakob disease (CJD); Fatal familial insomnia (FFI); Gerstmann-Straussler disease (GSD); Huntington disease-like 1 (HDL1); Kuru (KURU); Spongiform encephalopathy with neuropsychiatric features (SENF)
-
亚细胞定位:Cell membrane; Lipid-anchor, GPI-anchor. Golgi apparatus.
-
蛋白家族:Prion family
-
数据库链接:
HGNC: 9449
OMIM: 123400
KEGG: hsa:5621
STRING: 9606.ENSP00000368752
UniGene: Hs.472010
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-