Phospho-VASP (S157) Antibody
-
货号:CSB-PA060046
-
规格:¥880
-
其他:
产品详情
-
Uniprot No.:P50552
-
基因名:
-
别名:Vasodilator stimulated phosphoprotein antibody; Vasodilator-stimulated phosphoprotein antibody; VASP antibody; VASP_HUMAN antibody
-
宿主:Rabbit
-
反应种属:Human,Mouse,Rat
-
免疫原:Synthesized peptide derived from Human VASP around the phosphorylation site of S157.
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
-
抗体亚型:IgG
-
纯化方式:The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
-
产品提供形式:Liquid
-
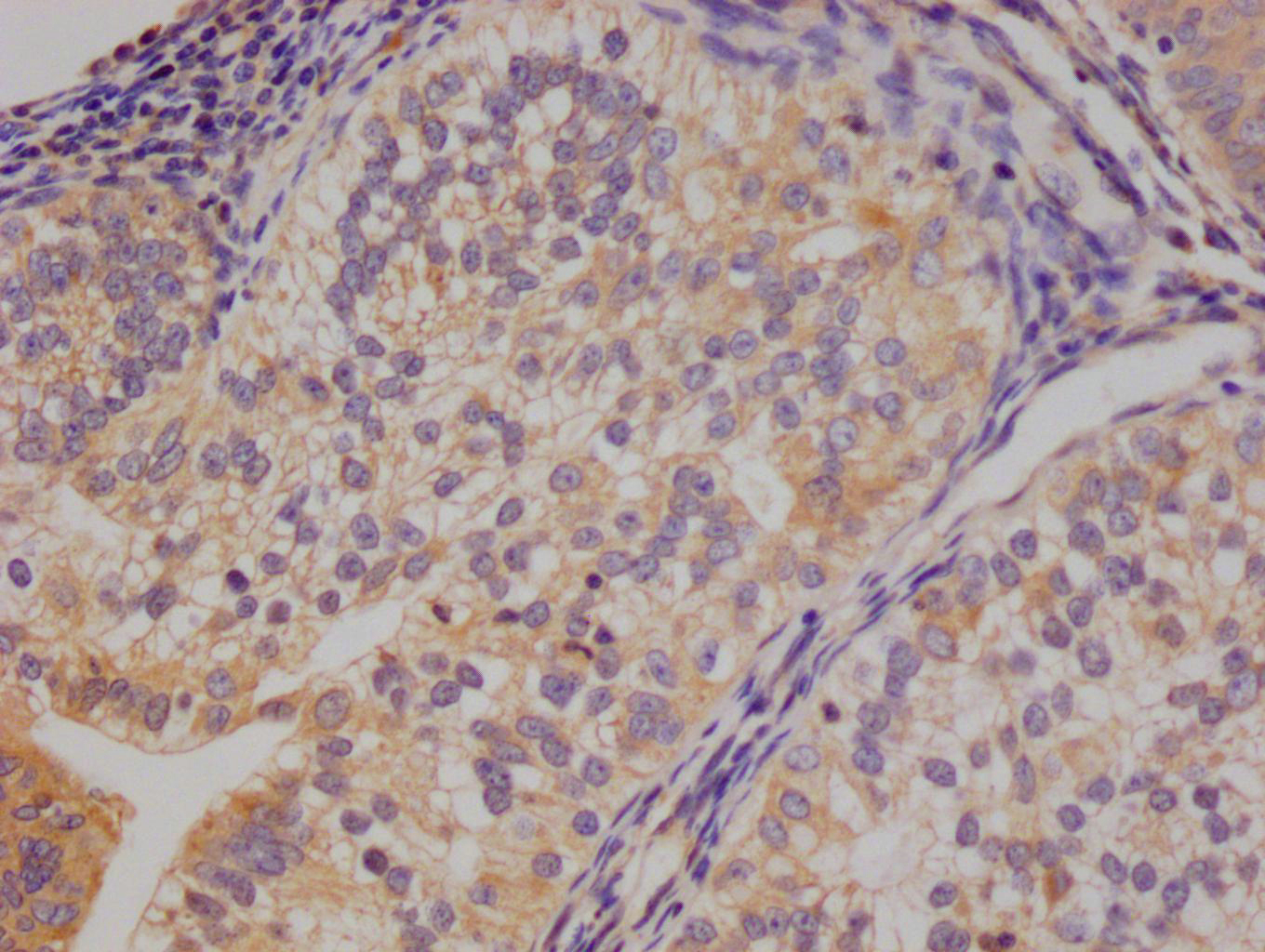
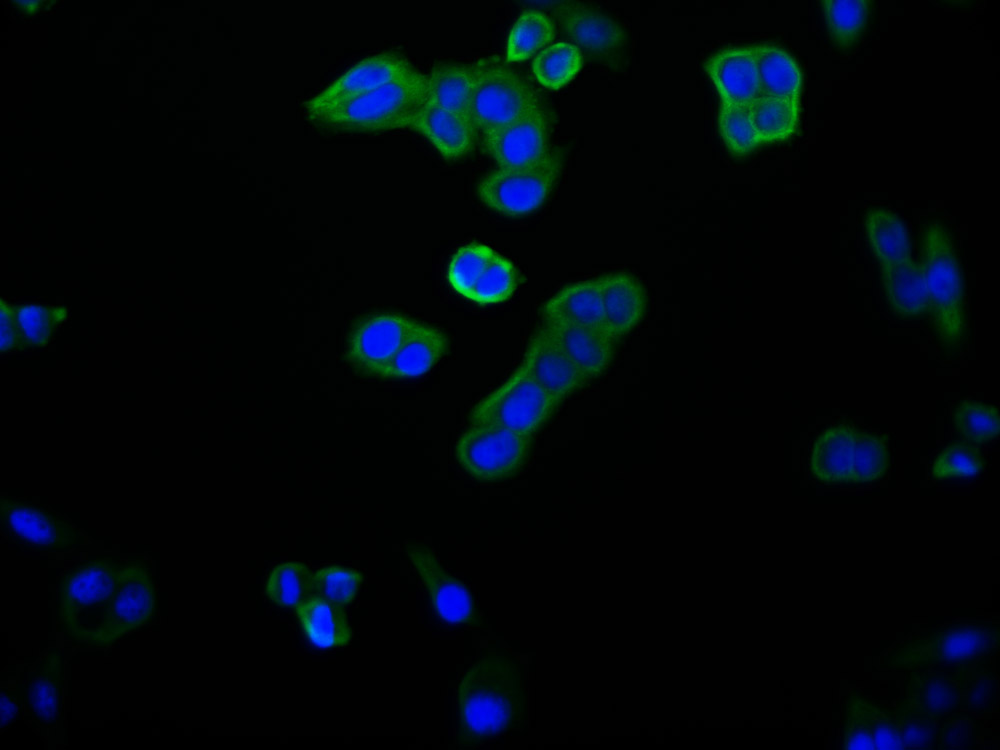
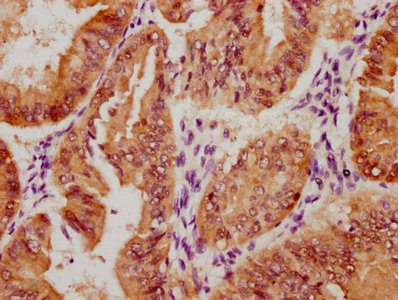
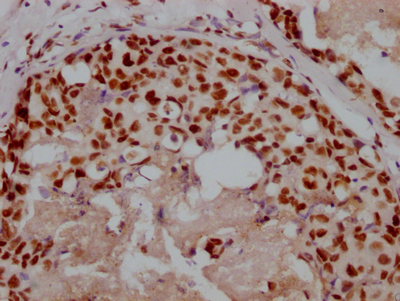
应用范围:WB, IHC, ELISA
-
推荐稀释比:
Application Recommended Dilution WB 1:500-1:2000 IHC 1:100-1:300 ELISA 1:5000 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Ena/VASP proteins are actin-associated proteins involved in a range of processes dependent on cytoskeleton remodeling and cell polarity such as axon guidance, lamellipodial and filopodial dynamics, platelet activation and cell migration. VASP promotes actin filament elongation. It protects the barbed end of growing actin filaments against capping and increases the rate of actin polymerization in the presence of capping protein. VASP stimulates actin filament elongation by promoting the transfer of profilin-bound actin monomers onto the barbed end of growing actin filaments. Plays a role in actin-based mobility of Listeria monocytogenes in host cells. Regulates actin dynamics in platelets and plays an important role in regulating platelet aggregation.
-
基因功能参考文献:
- VASP role in the actin filament elongation PMID: 28667124
- Our results reveal a dual role of VASP in endothelial permeability. In addition to its well-documented function in barrier integrity, we show that S-nitrosylation of VASP contributes to the onset of endothelial permeability. PMID: 28526707
- findings have uncovered a PKG/VASP signaling pathway in Vascular Smooth Muscle Cells as a key molecular mechanism underlying T3-induced vascular relaxation. PMID: 28376489
- This study provides the first evidence of VASP manipulation by an intravacuolar bacterial pathogen PMID: 27711191
- VASP silencing downregulated Migfilin, beta-catenin and uPA and impaired spheroid invasion. PMID: 28209486
- VASP phosphorylation assay could be useful in studies aimed at investigating relations between clopidogrel active metabolite bioavailability and clinical events. PMID: 26576037
- VASP, zyxin and TES are tension-dependent members of focal adherens junctions independent of the alpha-catenin-vinculin module. PMID: 26611125
- Data show that the phosphorylation status of vasodilator-stimulated phosphoprotein (VASP) at serine S322 can be predictive for breast cancer progression to an aggressive phenotype. PMID: 26336132
- The s propose that Lpd delivers Ena/VASP proteins to growing barbed ends and increases their actin polymerase activity by tethering them to actin filaments. PMID: 26295568
- Data show that tumor necrosis factor-alpha (TNF-alpha) increased A549 lung adenocarcinoma cell permeability by repressing vasodilator-stimulated phosphoprotein (VASP) expression through the activation of hypoxia inducible factor 1 alpha subunit (HIF-1alpha). PMID: 25051011
- The s demonstrate that vasodilator-stimulated phosphoprotein (VASP), which is critical for regulation of actin assembly, cell adhesion and motility, is a direct substrate of Yersinia pestis YpkA kinase activity. PMID: 25298072
- Ena/VASP's ability to bind F-actin and profilin-complexed G-actin are important for its effect, whereas Ena/VASP tetramerization is not necessary. PMID: 25355952
- In clinical practice,LCR and CYP2C19 gene polymorphism should be assessed in NCIS patients receiving clopidogrel treatment. PMID: 25457586
- VASP phosphorylation at Ser(157) mediates its localization at the membrane, but that VASP Ser(157) phosphorylation and membrane localization are not sufficient to activate its actin catalytic activity PMID: 25759389
- PKA regulates VASP phosphorylation in Ras-transformed cells in a non-cell-autonomous manner. PMID: 24963131
- Serine phosphorylation of vasodilator-stimulated phosphoprotein (VASP) regulates colon cancer cell survival and apoptosis. PMID: 25543053
- VASP reconstitution of actin-based motility depends on the recruitment of F-actin seeds from the solution produced by cofilin PMID: 25246528
- low serum concentration of vaspin is a risk factor for the progression of T2DM PMID: 24732788
- palladin functions as a dynamic scaffolding protein that promotes the assembly of dorsal stress fibers by recruiting VASP to these structures. PMID: 24496446
- Overexpression of VASP in endothelial cells blocked inflammation and insulin resistance induced by palmitate. PMID: 25117404
- Results show that NPs, possibly through the clearance receptor (natriuretic peptide receptor-C) expressed on platelet membranes, increase VASP phosphorylation but only following PDE inhibition, indicating a small, localised cGMP synthesis. PMID: 23469931
- Binding of tetrameric VASP to interleukin-1 receptor-associated kinase (IRAK)1 is regulated by assembly of IRAK1 onto signaling complexes. PMID: 24857403
- Matrine modulates the structure, subcellular distribution, expression and phosphorylation of VASP in human gastric cancer cells, thus inhibiting cancer cell adhesion and migration. PMID: 23685951
- PKD1 directly phosphorylates VASP at two serine residues, Ser-157 and Ser-322. These phosphorylations occur in response to RhoA activation and mediate VASP re-localization from focal contacts to the leading edge region. PMID: 23846685
- Letter: In High on-treatment platelet reactivity assessed by various platelet function tests the consensus-defined cut-off of VASP-P platelet reactivity index too low. PMID: 22212857
- Active proteases in nephrotic plasma lead to a podocin-dependent phosphorylation of VASP in podocytes via protease activated receptor-1. PMID: 23436459
- VASP participates in the regulation of cell cytoskeleton reorganization and morphology modification induced by shear flow via a cAMP/cAK pathway. PMID: 21158099
- a novel TNF-alpha/HIF-1alpha/VASP axis in which HIF-1alpha acts downstream of TNF-alpha to inhibit VASP expression and modulate the adhesion and proliferation of breast cancer cells PMID: 22320863
- Membrane organelle disassembly reflected specific phosphorylation of VASP Ser239, the cGMP/PKG preferred site, and rapid VASP removal from tumor cell protrusions PMID: 21702043
- Prolonged treatment with albuterol prevents the agonist-induced phosphorylation of VASP at Ser157. PMID: 22210825
- The phosphorylation and dephosphorylation cycle of VASP by the Abi-1-bridged mechanism regulates association of VASP with focal adhesions, which may regulate adhesion of Bcr-Abl-transformed leukaemic cells. PMID: 22014333
- The Ser-239 phosphorylation level of VASP might be a useful protein marker for riboflavin and UV light-mediated PLT compromise. PMID: 21827504
- ENA/VASP-family proteins are functionally redundant in homologous recombination, and MENA, VASP and EVL may be involved in the DSB repair pathway in humans PMID: 21398369
- VASP protein regulates osteosarcoma cell migration and metastasis PMID: 21874265
- Studied generation of filopodia with regards to the dynamic interaction established by Eps8, IRSp53 and VASP with actin filaments. PMID: 21814501
- Data show that VASP and Mena interact with RSK1. PMID: 21423205
- Data show that VASP has different immunostaining patterns between cerebral cortical plates in prenatal and adult human brain samples, and suggest that VASP may play a crucial role in the regulation of human neonatal cerebral cortical development. PMID: 21163344
- VASP deficiency leads to a more profound endothelial barrier disruption and delayed recovery after activation of thrombin PAR-1 receptor. PMID: 20945373
- vasodilator-stimulated phosphoprotein is phosphorylated in patients with genetic defects of the platelet P2Y(12) receptor for ADP PMID: 20695985
- High VASP expression is associated with focal adhesion assembly in myofibroblasts fostering a microenvironment that promotes tumor growth. PMID: 20802179
- Results describe the impact of smoking on platelet reactivity and phosphorylation of vasodilator-stimulated phosphoprotein (VASP) in a group of 20 young smokers. PMID: 20822337
- Results suggest that actin polymerization and bundling by VASP are critical for spine formation, expansion, and modulating synaptic strength. PMID: 20826790
- peroxynitrite may inhibit platelet function by inducing the phosphorylation of VASP through a mechanism that requires the activation of PKC. PMID: 20624010
- presentation of a model for how VASP promotes actin filament assembly PMID: 21041447
- Letter: Report vasodilator-stimulated phosphoprotein (VASP) ELISA to evaluate P2Y12-ADP receptor activity in coronary artery disease patients taking antiplatelet agents. PMID: 20589315
- Platelet hyperreactivity in multiple electrode aggregometry might be a better risk predictor for stent thrombosis than the assessment of the specific clopidogrel effect with the VASP phosphorylation assay. PMID: 19943879
- Combination of experimental and computational interactome research was used for the analysis of protein-protein interactions between Abi-1 and VASP in human platelets. PMID: 20110575
- Compared clopidogrel effectiveness in unstable ST-elevation myocardial infarction (STEMI) patients on mechanical ventilation with stable STEMI patients using the VASP index. PMID: 19902490
- IRAK-1 forms a close complex with PKCepsilon as well as VASP, and participates in phorbol 12-myristate 13-acetate-induced phosphorylation of VASP. PMID: 20044140
- VASP phosphorylation controls remodeling of the actin cytoskeleton. PMID: 19825941
显示更多
收起更多
-
亚细胞定位:Cytoplasm. Cytoplasm, cytoskeleton. Cell junction, focal adhesion. Cell junction, tight junction. Cell projection, lamellipodium membrane. Cell projection, filopodium membrane. Note=Targeted to stress fibers and focal adhesions through interaction with a number of proteins including MRL family members. Localizes to the plasma membrane in protruding lamellipodia and filopodial tips. Stimulation by thrombin or PMA, also translocates VASP to focal adhesions. Localized along the sides of actin filaments throughout the peripheral cytoplasm under basal conditions. In pre-apoptotic cells, colocalizes with MEFV in large specks (pyroptosomes).
-
蛋白家族:Ena/VASP family
-
组织特异性:Highly expressed in platelets.
-
数据库链接:
HGNC: 12652
OMIM: 601703
KEGG: hsa:7408
STRING: 9606.ENSP00000245932
UniGene: Hs.515469
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-