TNNC1 Antibody
-
货号:CSB-PA024009LA01HU
-
规格:¥440
-
促销:
-
图片:
-
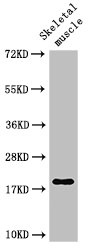
Western Blot
Positive WB detected in: Rat skeletal muscle tissue
All lanes: TNNC1 antibody at 3μg/ml
Secondary
Goat polyclonal to rabbit IgG at 1/50000 dilution
Predicted band size: 19 kDa
Observed band size: 19 kDa -
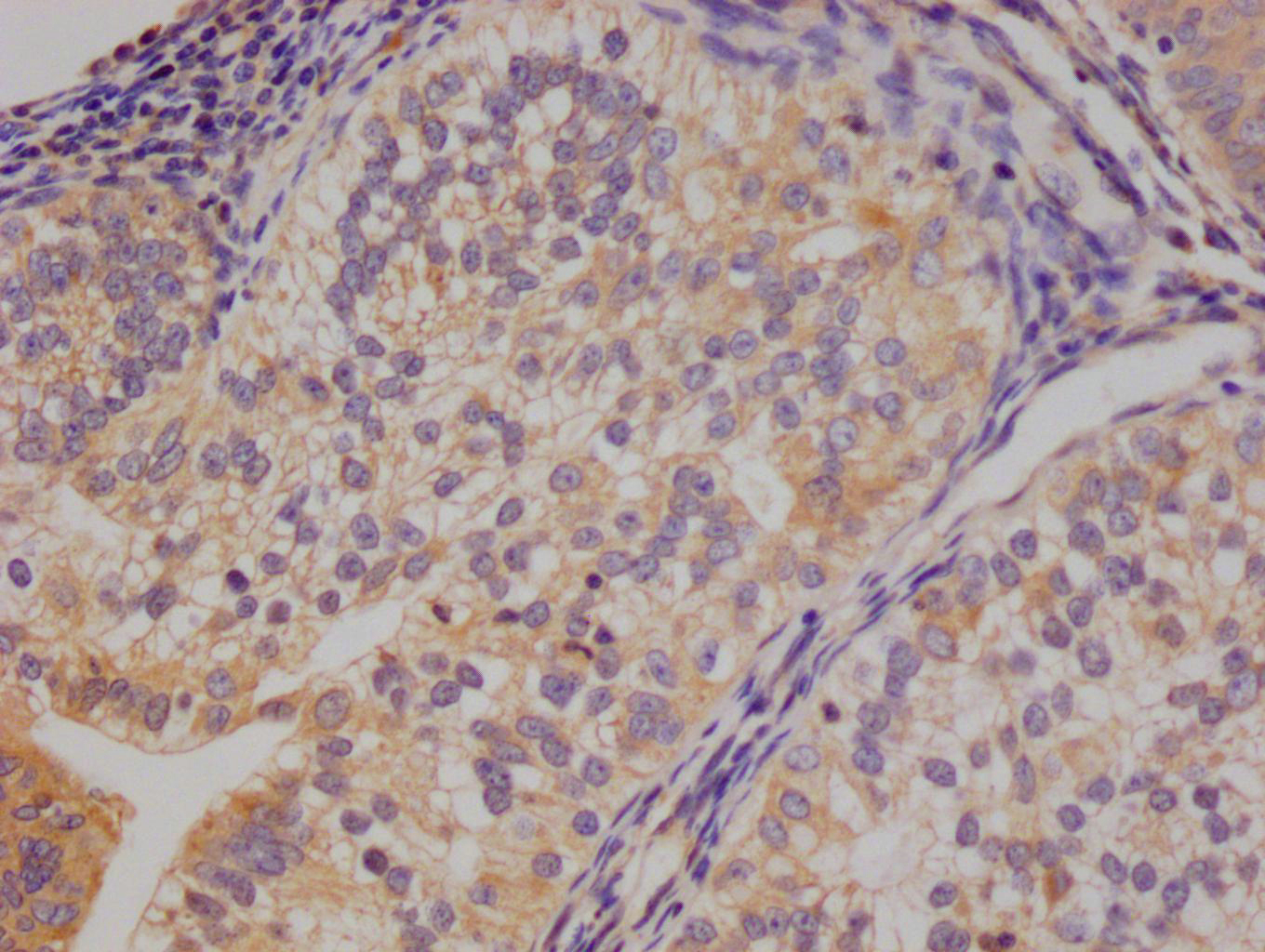
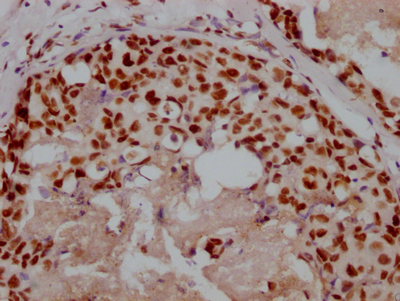
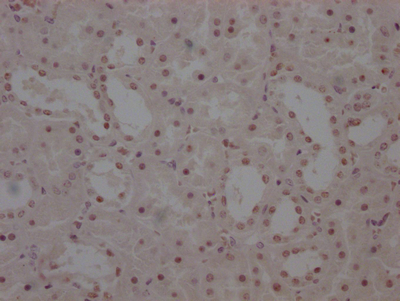
Immunohistochemistry of paraffin-embedded human heart tissue using CSB-PA024009LA01HU at dilution of 1:100
-
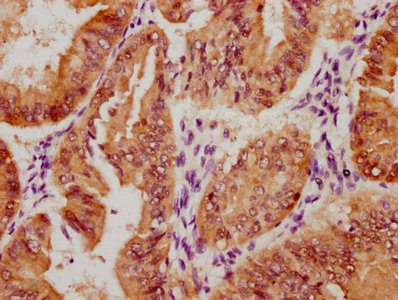
Immunohistochemistry of paraffin-embedded human skeletal muscle tissue using CSB-PA024009LA01HU at dilution of 1:100
-
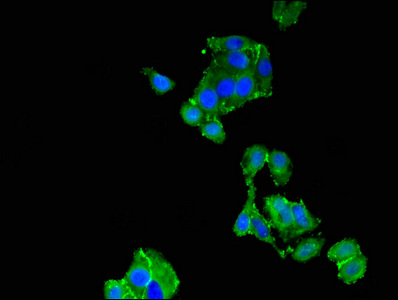
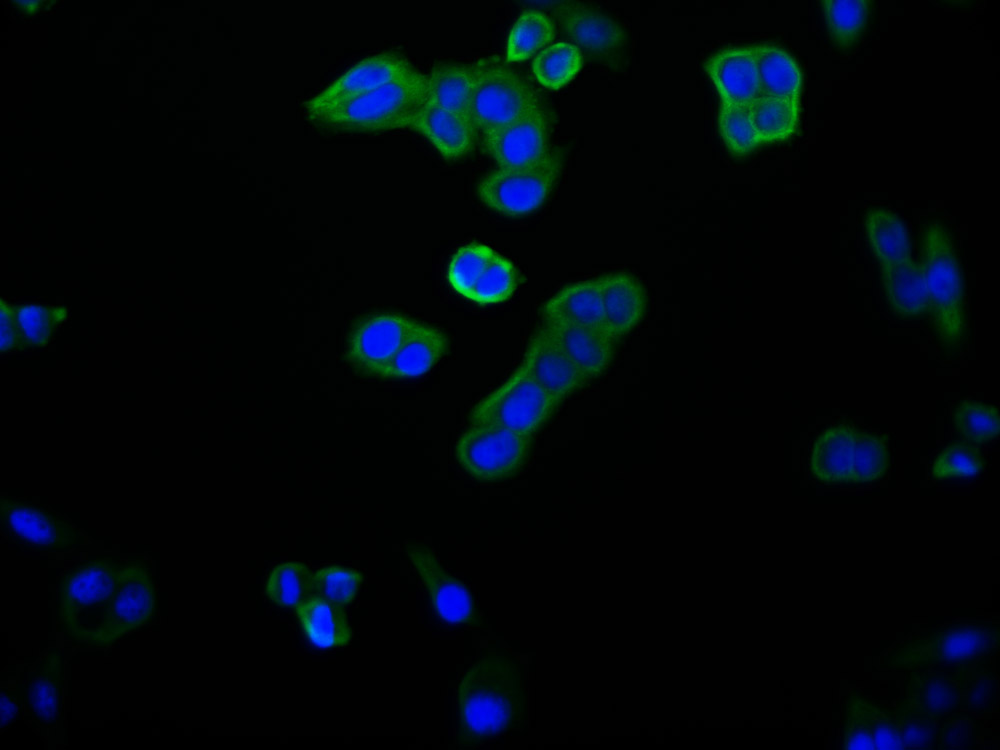
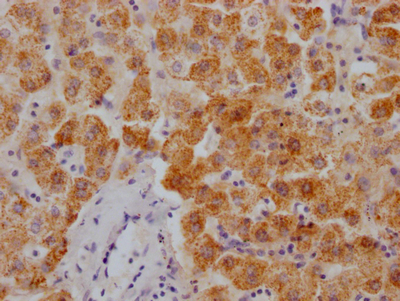
Immunofluorescent analysis of HepG2 cells using CSB-PA024009LA01HU at dilution of 1:100 and Alexa Fluor 488-congugated AffiniPure Goat Anti-Rabbit IgG(H+L)
-
-
其他:
产品详情
-
产品名称:Rabbit anti-Homo sapiens (Human) TNNC1 Polyclonal antibody
-
Uniprot No.:P63316
-
基因名:
-
别名:tnnc1a antibody; Cardiac troponin C antibody; slow skeletal and cardiac muscles antibody; TN-C antibody; TNC antibody; Tnnc1 antibody; TNNC1_HUMAN antibody; TNNI3 antibody; Troponin C antibody; Troponin C slow antibody; Troponin C1 slow antibody
-
宿主:Rabbit
-
反应种属:Human, Rat
-
免疫原:Recombinant Human Troponin C, slow skeletal and cardiac muscles protein (11-149AA)
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
本页面中的产品,TNNC1 Antibody (CSB-PA024009LA01HU),的标记方式是Non-conjugated。对于TNNC1 Antibody,我们还提供其他标记。见下表:
-
克隆类型:Polyclonal
-
抗体亚型:IgG
-
纯化方式:>95%, Protein G purified
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:Preservative: 0.03% Proclin 300
Constituents: 50% Glycerol, 0.01M PBS, pH 7.4 -
产品提供形式:Liquid
-
应用范围:ELISA, WB, IHC, IF
-
推荐稀释比:
Application Recommended Dilution WB 1:500-1:5000 IHC 1:20-1:200 IF 1:50-1:200 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Troponin is the central regulatory protein of striated muscle contraction. Tn consists of three components: Tn-I which is the inhibitor of actomyosin ATPase, Tn-T which contains the binding site for tropomyosin and Tn-C. The binding of calcium to Tn-C abolishes the inhibitory action of Tn on actin filaments.
-
基因功能参考文献:
- Rationally engineered TnC constructs corrected the abnormal Ca(2+) sensitivities of the thin filament, reconstituted actomyosin ATPase activity PMID: 22511780
- Molecular effects of cardiac troponin C mutations present in hypertrophic cardiomyopathy on calcium sensitivity and myofilament activation have been reported. PMID: 27133568
- Study shows that over-expression of MFAP5 and TNNC1 is correlated with cervical lymph node metastasis (CLNM), metastasis relapse-free survival and overall survival. These results propose that MFAP5 and TNNC1 may be potential markers for predicting occult cervical lymphatic metastasis and prognosis of oral tongue carcinoma. PMID: 27713166
- Our results (i) confirm that genetic backgrounds of hypertrophic cardiomyopathy and restrictive cardiomyopathy overlap and (ii) indicate that TNNC1 is a likely novel gene for autosomal recessive restrictive cardiomyopathy. PMID: 27604170
- Data suggest that modulation of structural dynamics far from the regulatory Ca2+-binding site is the underlying molecular mechanism for many TNNC1 mutations in patients with hypertrophic cardiomyopathies or familial hypertrophic cardiomyopathies; many mutations affect balance between open and closed conformations; troponin I switch peptide [TnI(SW)] switch peptide binds to TNNC1 and stabilizes the open TNNC1 conformation. PMID: 28533433
- Data suggest that mutations in troponin C (TnC) found in patients with hypertrophic cardiomyopathy (A8V, C84Y, and D145E) stabilize the active state of regulated actin (the actin-tropomyosin-troponin complex) to various extents; at a saturating Ca2+ concentration, all TnC mutants investigated increase the level of active M state compared to the wild type. PMID: 28530094
- There was no difference in the test characteristics of the HEART Pathway whether using cTnI or hs-cTnI, with both achieving 100% sensitivity and NPV. Use of hs-cTnT with the HEART Pathway was associated with one missed major adverse cardiac events. PMID: 28087371
- contractility is constantly above normal in hearts made hypertrophic by TnC with the A8V mutation PMID: 26976709
- We used nuclear magnetic resonance and circular dichroism to solve the structure and characterize the backbone dynamics and stability of the regulatory domain of cTnC with the L29Q mutation. PMID: 26341255
- conclusive evidence that TNNC1 is an uncommon but definitive HCM-susceptibility gene PMID: 26304555
- Troponin C (TnC) and the N-terminal helix of Troponin I (TnI N-helix), which occurs in vivo during muscle contraction. PMID: 26111167
- FAK/CREB/TNNC1 has a role in mediating the effect of stromal MFAP5 on ovarian cancer metastatic potential PMID: 25277212
- Mutations in cTnC have been associated with hypertrophic or dilated cardiomyopathy.[review] PMID: 26232335
- Data suggest that mutation A162H in switch region of troponin I induces transitory curved conformation and promotes contraction of troponin I bound to regulatory domain of troponin C; this is countered by residue E164 to ensure proper relaxation. PMID: 25996354
- in vitro characterisation of six cardiac Troponin C mutations causing hypertrophic and dilated cardiomyopathies (Review) PMID: 24744096
- The conformational dynamics of N-terminal lobe of TnC plays an important role in the regulation of cardiac muscle contraction. PMID: 25101951
- Data indicate that domain positioning impacts the effective concentration of cardiac isoform of troponin I (cTnI) presented to cardiac troponin C (cTnC). PMID: 25246568
- Toponin I, T, and C play crucial roles in muscle activity, connecting changes in intracellular Ca2+ concentration with generation of contraction. [review] PMID: 24490734
- Central helix point mutations decreased affinity of Ca2+ saturated cardiac TNC for TnI128-180. PMID: 24650606
- The structure of cardiac troponin C regulatory domain with bound Cd2+ reveals a closed conformation and unique ion coordination. PMID: 23633581
- Calcium induced regulation of skeletal troponin--computational insights from molecular dynamics simulations. PMID: 23554884
- calcium binding to the regulatory site of human cardiac troponin C PMID: 23111626
- Significance of troponin dynamics for Ca2+-mediated regulation of contraction and inherited cardiomyopathy. PMID: 23066014
- a novel mutation in the TNNC1 gene is associated with HCM pathogenesis and may predispose to the pathogenesis of a fatal arrhythmogenic subtype of HCM PMID: 22815480
- The study examines TNC for its ability of binding Ca2+ and furthermore determines the molecular contributions to Ca2+ binding kinetics. PMID: 22329450
- The L48Q mutation enhanced binding of both Ca(2+) and troponin I to cardiac troponin C. PMID: 22591429
- The disease-related protein modifications alter Ca(2+) binding by influencing both the association and dissociation rates of thin filament Ca(2+) exchange. PMID: 22675533
- Cardiomyopathy-linked TnC mutations affect the response of reconstituted thin filaments to calcium upon cardiac troponin (Tn)I phosphorylation. PMID: 22489623
- Functional characterization of TNNC1 rare variants identified in dilated cardiomyopathy. PMID: 21832052
- strong cross-bridges potentiate the Ca(2+)-sensitizing effect of hypertrophic cardiomyopathy-cTnC mutants on the myofilament PMID: 21056975
- analysis of order and disorder in troponin C, T and I PMID: 20889975
- A region in cTnC associated with increased Ca(2+) sensitivity in skinned fibers was identified, an the F27W reporter mutation affected Ca(2+) sensitivity, maximal force, and ATPase activation of some mutants. PMID: 20566645
- plasma levels are associated with degree of vascular obstruction in patients with pulmonary embolism PMID: 19492165
- Calcium binding properties of the carboxy terminal-domain sites might be important for the proper regulatory function of cardiac troponin C. PMID: 20459070
- Four private protein-altering variants were identified in troponin C type 1 in 4 probands. PMID: 20215591
- After acute myocardial infarction, cTnI is present in serum as the ternary cTnT-cTnI-TnC (TIC) complex and binary cTnI-TnC (IC) complex. PMID: 20378771
- the dilated cardiomyopathy troponin C mutation lowers contractile force by reducing strong myosin-actin binding PMID: 20371872
- The intrinsic properties of TnC and its interactions with other contractile proteins play a crucial role in modulating the binding of calcium to TnC in increasingly complex biochemical systems. PMID: 20128626
- cardiac troponin switches between alternative sets of intramolecular interactions, similar to previous intermediate resolution x-ray data of skeletal muscle troponin PMID: 19920153
- Structure and dynamics of the C-domain of human cardiac troponin C in complex with the inhibitory region of human cardiac troponin I. PMID: 12732641
- Data suggest that activation of cardiac myofilaments is tightly coupled to the open state of the N-domain of cardiac troponin C, and that pathological effects of phosphorylation are influenced by mutations in cardiac troponin I. PMID: 15147183
- CTnI mutations mainly alter myocardial performance via changes in the Ca2+ -sensitivity of force development and in some cases alter the muscle relaxation kinetics. Review. PMID: 15524171
- C helix moves away from the D helix in a distinct Ca(2+)-dependent manner, while the B helix does not. PMID: 15628883
- Results describe the in situ structure of human cardiac troponin C. PMID: 15808858
- The crystal structure of troponin suggests that the Ca2+-binding to the regulatory TnC site displaces the N-terminal portion of TnI from actin/tropomyosin, thereby altering mobility/flexibility of the troponin/tropomyosin strand on the actin filament. PMID: 16157639
- Spin dipole-dipole interaction showed that in reconstituted muscle fibers both skeletal and cardiac TnC undergo Ca2+-induced structural change that is thought to be TnIreg movement. PMID: 16157641
- Results imply a hindered transduction of the protein kinase A phosphorylation signal from cardiac troponin I to troponin C. PMID: 16302972
- the mutation Gly159Asp causes a significant decrease in the rate of force production and a change in the relationship between the rate of force production and generated force in muscle PMID: 17021793
- in the presence of phosphorylated cTnI, cTnC-G159D specifically blunted phosphorylation induced decrease in Ca(2+)-sensitive tension development without altering cross-bridge cycling in cardiac myofilament PMID: 17446435
- Suggest that TnC Ca(2+) binding properties modulate the rate of cardiac muscle contraction at submaximal levels of Ca(2+) activation. PMID: 17693547
显示更多
收起更多
-
相关疾病:Cardiomyopathy, dilated 1Z (CMD1Z); Cardiomyopathy, familial hypertrophic 13 (CMH13)
-
蛋白家族:Troponin C family
-
数据库链接:
HGNC: 11943
OMIM: 191040
KEGG: hsa:7134
STRING: 9606.ENSP00000232975
UniGene: Hs.118845
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-