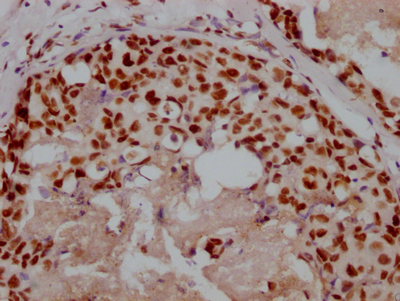
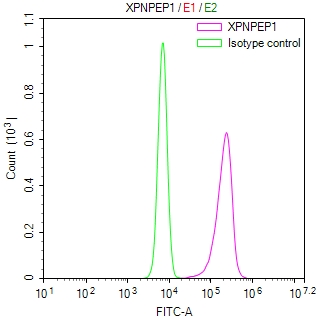
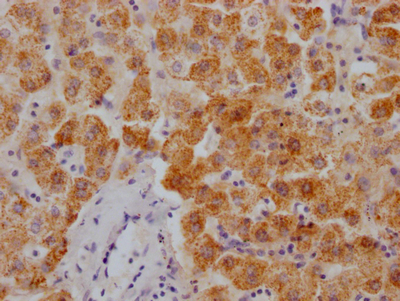
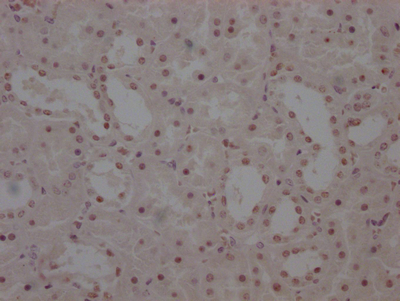
UBC Antibody
-
货号:CSB-PA03742A0Rb
-
规格:¥440
-
促销:
-
图片:
-
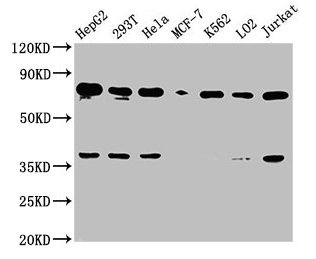
Western Blot
Positive WB detected in: HepG2 whole cell lysate, 293T whole cell lysate, Hela whole cell lysate, MCF-7 whole cell lysate, K562 whole cell lysate, LO2 whole cell lysate, Jurkat whole cell lysate
All lanes: UBC antibody at 1:2000
Secondary
Goat polyclonal to rabbit IgG at 1/50000 dilution
Predicted band size: 78 kDa
Observed band size: 78 kDa
-
-
其他:
产品详情
-
产品名称:Rabbit anti-Homo sapiens (Human) UBC Polyclonal antibody
-
Uniprot No.:P0CG48
-
基因名:
-
别名:UBC antibody; Polyubiquitin-C [Cleaved into: Ubiquitin] antibody
-
宿主:Rabbit
-
反应种属:Human
-
免疫原:Peptide sequence from Human Polyubiquitin-C protein (19-36AA)
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
本页面中的产品,UBC Antibody (CSB-PA03742A0Rb),的标记方式是Non-conjugated。对于UBC Antibody,我们还提供其他标记。见下表:
-
克隆类型:Polyclonal
-
抗体亚型:IgG
-
纯化方式:Antigen Affinity Purified
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:Preservative: 0.03% Proclin 300
Constituents: 50% Glycerol, 0.01M PBS, pH 7.4 -
产品提供形式:Liquid
-
应用范围:ELISA, WB
-
推荐稀释比:
Application Recommended Dilution WB 1:1000-1:5000 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Exists either covalently attached to another protein, or free (unanchored). When covalently bound, it is conjugated to target proteins via an isopeptide bond either as a monomer (monoubiquitin), a polymer linked via different Lys residues of the ubiquitin (polyubiquitin chains) or a linear polymer linked via the initiator Met of the ubiquitin (linear polyubiquitin chains). Polyubiquitin chains, when attached to a target protein, have different functions depending on the Lys residue of the ubiquitin that is linked: Lys-6-linked may be involved in DNA repair; Lys-11-linked is involved in ERAD (endoplasmic reticulum-associated degradation) and in cell-cycle regulation; Lys-29-linked is involved in lysosomal degradation; Lys-33-linked is involved in kinase modification; Lys-48-linked is involved in protein degradation via the proteasome; Lys-63-linked is involved in endocytosis, DNA-damage responses as well as in signaling processes leading to activation of the transcription factor NF-kappa-B. Linear polymer chains formed via attachment by the initiator Met lead to cell signaling. Ubiquitin is usually conjugated to Lys residues of target proteins, however, in rare cases, conjugation to Cys or Ser residues has been observed. When polyubiquitin is free (unanchored-polyubiquitin), it also has distinct roles, such as in activation of protein kinases, and in signaling.
-
基因功能参考文献:
- Regulation of transcriptional activators by DNA-binding domain (DBD) ubiquitination has shown that, when attached to the DBD of either p53 or IRF-1, ubiquitin is orientated towards, and makes contact with, the DNA. PMID: 28362432
- Data show that ubiquitin variants (Ubvs) that bind to USP2 or USP21 contain a similar core functional epitope, or "hot spot," consisting mainly of positions that are conserved as the wild type sequence, but also some positions that prefer mutant sequences. PMID: 27436899
- Data show that the packing of ubiquitin can significantly alter the thermodynamics and kinetics of local conformational exchange. PMID: 28747759
- Model of ASB9 in complex with its substrate, creatine kinase, reveals a mechanism for dynamic ubiquitin transfer. PMID: 27396830
- The lysine48-lysine63 branched ubiquitin chain regulates NF-kappaB signaling. PMID: 27746020
- Noncovalent ubiquitin interactions regulate the catalytic activity of ubiquitin writers. (Review) PMID: 27614784
- The s now report the crystal structure of a human Parkin-phosphoubiquitin complex, which shows that phosphoubiquitin binding induces movement in the 'in-between RING' (IBR) domain to reveal a cryptic ubiquitin-binding site. PMID: 28414322
- Studies indicate a role for the ubiquitin-proteasome system (UPS) as a key regulator of ciliogenesis. PMID: 27911708
- Chicago Sky Blue 6B (CSB6B) binds directly to the beta-groove of ubiquitin and could inhibit the binding of ubiquitin to chemokine (C-X-C motif) receptor 4 (CXCR4), a cell surface ubiquitin receptor. PMID: 27613091
- Studies suggest that ubiquitin signals for the proteasome involved more that Lys48 (K48). PMID: 28069863
- Data show that the stereospecific complex of ubiquitin and the ubiquitin-associated domain (UBA) is minimally perturbed by the crowding agent Ficoll. PMID: 28267209
- Studies indicate complex ubiquitin architectures function as important signals including post-translational modification (PTM) of ubiquitin itself, such as acetylated ubiquitin and phospho-ubiquitin.. PMID: 28011818
- Upon stabilization of the protein's core, the long loop converges on the core in the final step of the folding process. PMID: 27111887
- Our study predicted that UBC and RPA had potential as target genes for the diagnosis and treatment of osteosarcoma PMID: 26782416
- Data show that at least three heat shock elements with different configurations, exist in the UBC promoter. All of them are bound by transcription factors belonging to the heat shock factor family clarifying the mechanisms regulating UBC expression. PMID: 26317694
- Data show that discoidin domain receptor 2 (DDR2) is linked to a polyubiquitin (Ub) chain predominantly through lysine K27 conjugation and slightly through K33. PMID: 26271983
- USP4 requires its N-terminal DUSP-Ubl domain to achieve full catalytic turnover by promoting ubiquitin exchange. PMID: 25404403
- Data suggest that ubiquitin-conjugating enzyme E2 variant 2 (Ube2V2) and ring finger protein 4 (RNF4) together induce an active conformation of the ubiquitin-conjugating enzyme Ubc13-ubiquitin (Ubc13~Ub) thioester. PMID: 26148049
- noncovalent ubiquitin:ubiquitin interactions are nearly identical to those reported for Lys11-linked ubiquitin and seem to play a significant role in stabilizing the crystal structure without the isopeptide bond. PMID: 26171660
- Studies indicate that ubiquitin proteasome system (UPS) controls all aspects of cholesterol metabolism including its synthesis, uptake, and efflux. PMID: 25220377
- Studies suggest that quantitative proteomic approaches would set a standard for elucidating biochemical mechanisms of Ubiquitin (UB)-driven signaling systems. PMID: 26000850
- In testicular germ cell tumors, the ubiquitin expression is decreased indicating disturbances of ubiquitin-proteolysis system components at the initial stages of testicular tissue carcinogenesis. PMID: 26118027
- Data indicate that TRAF interacting protein TRIP negatively regulates the TNFR-associated factor 2 (TRAF2) ubiquitin-dependent pathway by modulating the TRAF2-sphingosine 1-phosphate (S1P) interaction. PMID: 25716317
- Data indicate that heat shock protein 90kDa (Hsp90) inhibition suppresses 26S proteasome remodeling, unanchored ubiquitin chain production, and aggresome clearance. PMID: 25713068
- Static HMG-20 structure is derived from high precision residual dipolar couplings measured in a drug-based liquid crystalline phase using NMR spectroscopy. PMID: 24568736
- This study identifies altered proteolysis as a feature of persistent podocyte injury. In the future, specific UPS proteins may serve as new biomarkers or therapeutic targets in persistent nephrotic syndrome. PMID: 24722446
- Data indicate that conditional replacement of endogenous ubiquitin (Ub) by Ub(R54A/Y59A) or Ub(K48R) yielded profound apoptosis at a similar extent. PMID: 24912152
- Data indicate that a single point deletion (DeltaE81) in RAP80 abrogates multivalent interactions with polyubiquitin. PMID: 24627472
- UbcH5c~Ubiqitin binding stabilizes an active conformation of the Shigella flexneri OspG kinase, greatly enhancing its activity. PMID: 24446487
- These results reveal an unanticipated mode of Ube2g2 self-association that allows Ube2g2 to effectively engage two ubiquitins to specifically synthesize Lys48-linked ubiquitin chains. PMID: 24366945
- Yin Yang 1 intronic binding sequences and splicing elicit intron-mediated enhancement of ubiquitin C gene expression. PMID: 23776572
- This article reviews the recent advances in proteomics of HMG20 and reveals novel networks and associations with human disease.[review] PMID: 23339974
- The donor ubiquitin, transferred from the E2, is bound to the Nedd4 C lobe with its C-terminal tail locked in an extended conformation, primed for catalysis. PMID: 23644597
- Ubiquitin's regulatory mechanisms of expression in heart failure patients' cardiomyocytes. (review) PMID: 23180530
- The GP78 CUE domain functions to both facilitate substrate binding and enable switching between adjacent ubiquitin molecules of a growing chain to enable processivity in ubiquitination. PMID: 23123110
- Data suggest that ubiquitin (Ub) binding provides a negative feedback loop upon NOD1 and NOD2 (nucleotide-binding oligomerization domain-containing proteins)-dependent activation of receptor-interacting protein kinase 2 (RIP2). PMID: 23300079
- Data indicate that pressure induced ubiquitin unfolding in methanol. PMID: 23284170
- Regulation of ubiquitin transfer by XIAP, a dimeric RING E3 ligase. PMID: 23259674
- The human ubiquitin C promoter transgene might be useful to selectively target projections of brain neurons. PMID: 21802467
- analysis of cold-induced changes in the protein ubiquitin PMID: 22737208
- Ubiquitin targeting of tau protein occurs at neurofibrillary tangles in the early and intermediate maturation stages. PMID: 21919991
- Data indicate that modification of NEMO with linear di-ubiquitin is sufficient for full NF-kappaB activation. PMID: 22605335
- the ubiquitin independent degradation pathway utilized by a hepatitis B virus envelope protein limits antigen presentation PMID: 21969857
- Studies indicate that signaling controlled by ubiquitin or ubiquitin-like proteins has recently emerged as key regulator of the cellular DNA damage response, and viruses can reveal key convergence points in this important cellular pathway. PMID: 21549706
- Studies indicate that Ku80 is removed from DNA through a ubiquitin-mediated process. PMID: 21640108
- Studies indicate that Non-proteolytic ubiquitylation of chromatin surrounding DSBs, mediated by the RNF8/RNF168 ubiquitin ligase cascade, has emerged as a key mechanism for restoration of genome integrity. PMID: 21664912
- Studies suggest that DNA damage-induced ubiquitination or sumoylation of PCNA prevents CRL4Cdt2-dependent degradation by inhibiting binding of Cdt1 to PCNA. PMID: 21846465
- Studies indicate that monoubiquitylation of PCNA allows mutagenic translesion synthesis by damage-tolerant DNA polymerases, polyubiquitylation is required mainly for an error-free pathway that likely involves template switching. PMID: 21605556
- Studies indicate that All the Y-family polymerases have ubiquitin binding domains that bind to mono-ubiquitinated PCNA to effect the switching from replicative to Y-family polymerase. PMID: 21704031
- Studies indicate that the involvement of the degradation-linked K48-ubiquitin signal and the proteasome at the sites of DSBs. PMID: 21536036
显示更多
收起更多
-
亚细胞定位:[Ubiquitin]: Cytoplasm. Nucleus.
-
蛋白家族:Ubiquitin family
-
数据库链接:
HGNC: 12468
OMIM: 191340
KEGG: hsa:7316
STRING: 9606.ENSP00000344818
UniGene: Hs.520348
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-