Recombinant Chicken Lysozyme C (LYZ)
-
中文名称:鸡LYZ重组蛋白
-
货号:CSB-YP013283CH
-
规格:
-
来源:Yeast
-
其他:
-
中文名称:鸡LYZ重组蛋白
-
货号:CSB-EP013283CH
-
规格:
-
来源:E.coli
-
其他:
-
中文名称:鸡LYZ重组蛋白
-
货号:CSB-BP013283CH
-
规格:
-
来源:Baculovirus
-
其他:
-
中文名称:鸡LYZ重组蛋白
-
货号:CSB-MP013283CH
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:LYZ
-
Uniprot No.:
-
别名:LYZLysozyme C; EC 3.2.1.17; 1,4-beta-N-acetylmuramidase C; Allergen Gal d IV; allergen Gal d 4
-
种属:Gallus gallus (Chicken)
-
蛋白长度:Full Length of Mature Protein
-
表达区域:19-147
-
氨基酸序列KV FGRCELAAAM KRHGLDNYRG YSLGNWVCAA KFESNFNTQA TNRNTDGSTD YGILQINSRW WCNDGRTPGS RNLCNIPCSA LLSSDITASV NCAKKIVSDG NGMNAWVAWR NRCKGTDVQA WIRGCRL
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Lysozymes have primarily a bacteriolytic function; those in tissues and body fluids are associated with the monocyte-macrophage system and enhance the activity of immunoagents. Has bacteriolytic activity against M.luteus.
-
基因功能参考文献:
- NG and NR inhibited the enzymatic activity of Hen Egg White Lysozyme (HEWL) and exhibited their affinity for the active site of HEWL. PMID: 29145059
- Molecular docking results suggested the location of vandetanib (VDB) binding site near the LYZ active site while molecular dynamics simulation results suggested stability of VDB-LYZ complex. PMID: 28843881
- Specific structural changes constitute the first steps in lysozyme unfolding by urea. PMID: 27573790
- Molecular dynamics (MD) simulation results demonstrate that the "hard protein" lysozyme retains much of its secondary structure during adsorption, whereas BSA loses it almost completely. BSA has a considerably larger adsorption energy compared to that of lysozyme, which does not scale with chain length. Desorption simulations are carried out using classical steered MD. PMID: 27421144
- The ability of an anion to slow down the water dynamics around lysozyme was found to have the following order: SCN- > Cl- > H2PO4- > NO3- approximately SO42-. This result indicates that the effects of anions on the dynamics of water around the lysozyme molecule are the opposite of those for bulk water. PMID: 27193313
- Protein microcrystals magnetically aligned in D2O hydrogels were subjected to neutron diffraction measurements, and reflections were observed for the first time to a resolution of 3.4 A from lysozyme microcrystals ( approximately 10 x 10 x 50 microm). PMID: 27377379
- Here, a single nanocrystal with a diffracting volume of only 0.14 microm(3), i.e. no more than 6 x 10(5) unit cells, provided sufficient information to determine the structure of a rare dimeric polymorph of hen egg-white lysozyme by electron crystallography. PMID: 28876237
- Solutions of lysozyme in heavy water were studied by small-angle neutron scattering at concentrations of 40, 20 and 10 mg ml(-1) with and without the addition of precipitant, and at temperatures of 10, 20 and 30 degrees C. In addition to the expected protein monomers, dimeric and octameric species were identified in solutions at the maximum concentration and close to the optimal conditions for crystallization. PMID: 28695859
- The enzyme activity of LYZ was inhibited by the addition of copper with catalytic residues Glu 35 and Asp 52 locating at the binding sites. This study helps to elucidate the molecular mechanism of the interaction between copper and lysozyme and provides reference for toxicological studies of copper. PMID: 27089183
- At neutral pH, lysozyme retains its native conformation between 0 and 8 M urea over the entire range of temperatures studied. PMID: 27933780
- Data show that hen lysozyme aggregates faster than the human lysozyme. PMID: 27825804
- Different C-type lysozyme gene haplotypes are associated with egg hatchability and survival in Rhode Island Red layer chickens PMID: 27478034
- Drospirenone had different effects on the local conformation of HSA and LYZ molecules. PMID: 26448295
- At micro-second and coarser temporal resolutions, free energy landscape of hen egg white lysozyme exhibits hub-like topology with crystal structures occupying the dominant structural ensemble. PMID: 26057625
- Data indicate that lysozyme significantly enhances the dewaterability of biosludge. PMID: 25462773
- Data show that copper(II) inhibits self-association of hen egg white lysozyme (HEWL) at pH 12.75 both at 37 and 65 degrees C. PMID: 24806136
- Under high pressure, the crystal structure of the enzyme undergoes several local and global changes accompanied by changes in hydration structure. PMID: 25849385
- Structure of hen egg-white lysozyme determined from single shots of trapped microcrystals. PMID: 25849403
- Egg white-lysozyme protein structure and stability revealed by X-ray crystallography. PMID: 25521080
- Underdamped delocalized vibrational modes in the terahertz frequency domain are identified and shown to blue-shift and strengthen upon LYZ-triacetylchitotriose binding. PMID: 24893252
- Data suggest that stabilization of lysozyme by mono- and oligo-saccharides can be accounted for by simplified statistical-thermodynamic model considering volume exclusion deriving from steric repulsion between enzyme and saccharides. PMID: 26000826
- These results show that pressure suppresses protein nucleation, aggregation, and finally crystallization in supersaturated condensed lysozyme solutions. PMID: 25494777
- The study explores fingerprinting the tertiary structure of electroadsorbed lysozyme at soft interfaces by electrostatic spray ionization ma.ss spectrometry PMID: 25156670
- Aberrant disulfide bonds in non-amyloidogenic proteins (like HEWL), may strengthen non-covalent intermolecular forces among monomers and promote their aggregation. PMID: 24551048
- This study compared the influence of Cu(II) ions and pH of the environment on the molecular structure of the native protein and its fibrils. PMID: 23786978
- lysozyme C structure determined by X-ray crystallography method PMID: 23295480
- This study analyzes binding structures of complexes of lysozyme and N-acetyl-beta-glucosamine trisaccharide, (NAG)(3). PMID: 23109228
- The results of the three simulations show that the structural properties of fully thionated LYZ clearly differ from those of the native protein, while for partly thionated LYZ they only changed slightly compared with native LYZ. PMID: 22653637
- Small-angle x-ray scattering studies on dense lysozyme solutions of high ionic strength as a function of temperature and pressure, are reported. PMID: 22713580
- Both backbone amide and side chain methyl bond vectors in LYZ are relatively rigid in both the apo and chitotriose-bound states. PMID: 22593013
- The tri-N-acetylglucosamine-bound state of the enzyme is less hydrated, more rigid, and less dynamic compared to the unbound state. PMID: 22732010
- The study provides a quantitative description of the solvation properties of lysozyme in water/ethanol mixtures, which has been obtained by a simultaneous analysis of small-angle neutron scattering and differential scanning calorimetry experiments. PMID: 22225188
- The study applied a protein mapping method to nine structures of hen egg-white lysozyme, and idenified ligand binding sites. PMID: 22092261
- investigation of protein conformation/stability of lysozyme by comparison with analogs with varying degrees of ester linkages replacing amide/peptide linkages using molecular dynamics simulations PMID: 22093234
- The study presents conformational responses of hen egg white lysozyme in the tetragonal crystal by X-ray diffraction experiments using a humidity-control apparatus, which provided air flow of 20-98%rh at 298 K. PMID: 21802827
- The interaction between gold nanorods and lysozyme has been monitored using spectroscopic techniques. PMID: 21729718
- These results demonstrate the enhanced chaperone activity of modified beta-casein and its protective effects on lysozyme refolding. PMID: 21802443
- The orientation of lysozyme adsorbed to a negatively charged ligand surface was predicted by a rigid and a flexible model. PMID: 21689536
- The whole hydration sites (HS) of lysozyme are composed of 195 single HSs and 38 clustered ones (CHS), and divided into 231 external HSs (EHS) and 2 internal ones (IHS). PMID: 21435773
- The NMR results indicate that low-lying excited state conformers of hen lysozyme are characterized by slowly fluctuating local conformations around these cavities, attributed to the opportunities for water molecules to penetrate into the cavities. PMID: 21367514
- fibril formation by hen egg white lysozyme PMID: 21483680
- Data revealed that lysozyme with higher SDS concentrations showed superior thermodynamic stabilities over the ones with no or lower levels of SDS. PMID: 20674294
- The reversible thermal unfolding of hen egg white lysozyme, was examined. PMID: 20923660
- The interactions of lysozyme with caffeine (Caf), theophylline (Tph) and theobromine (Tbr) were investigated. PMID: 19823947
- Results describe the size, shape, structure, and interactions of lysozyme in the ternary system lysozyme/DMSO/water at low protein concentrations. PMID: 20731407
- Utilizing fluorescence correlation spectroscopy high spatial resolution of about the laser wavelength used, the molecular dynamics close to crystal surfaces was investigated for both tetragonal single crystals and needlelike spherulites. PMID: 20831338
- Two model proteins, lysozyme and thaumatin, were used under unique flow conditions to differentially probe protein crystal nucleation and growth. PMID: 20713010
- Single crystal X-ray diffraction shows that the rhenium tricarbonyl cation binds to hen egg lysozyme His15 in two significantly populated rotamer conformations. PMID: 20449250
- Acetylation of the lysine residues promoted amyloid formation, resulting in more pronounced fibrils and a dramatic decline in the nucleation time. In contrast, citraconylation produced the opposite effect. PMID: 19945549
- The obtained data suggested that LSZ embedment within the H(II) mesophase improved its thermal stability by hampering its helical structure destruction, apparently due to hydrogen bonding of the protein with monoolein polar heads. PMID: 19836212
显示更多
收起更多
-
亚细胞定位:Secreted.
-
蛋白家族:Glycosyl hydrolase 22 family
-
组织特异性:In the egg white and polymorphonuclear leukocytes.
-
数据库链接:
KEGG: gga:396218
STRING: 9031.ENSGALP00000016177
UniGene: Gga.713
Most popular with customers
-
Recombinant Human IGF-like family receptor 1 (IGFLR1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
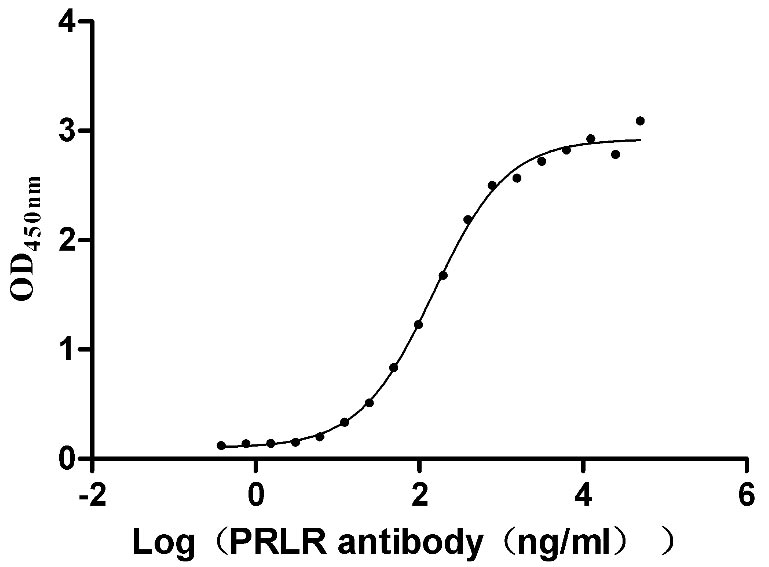
Recombinant Human Prolactin receptor (PRLR), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
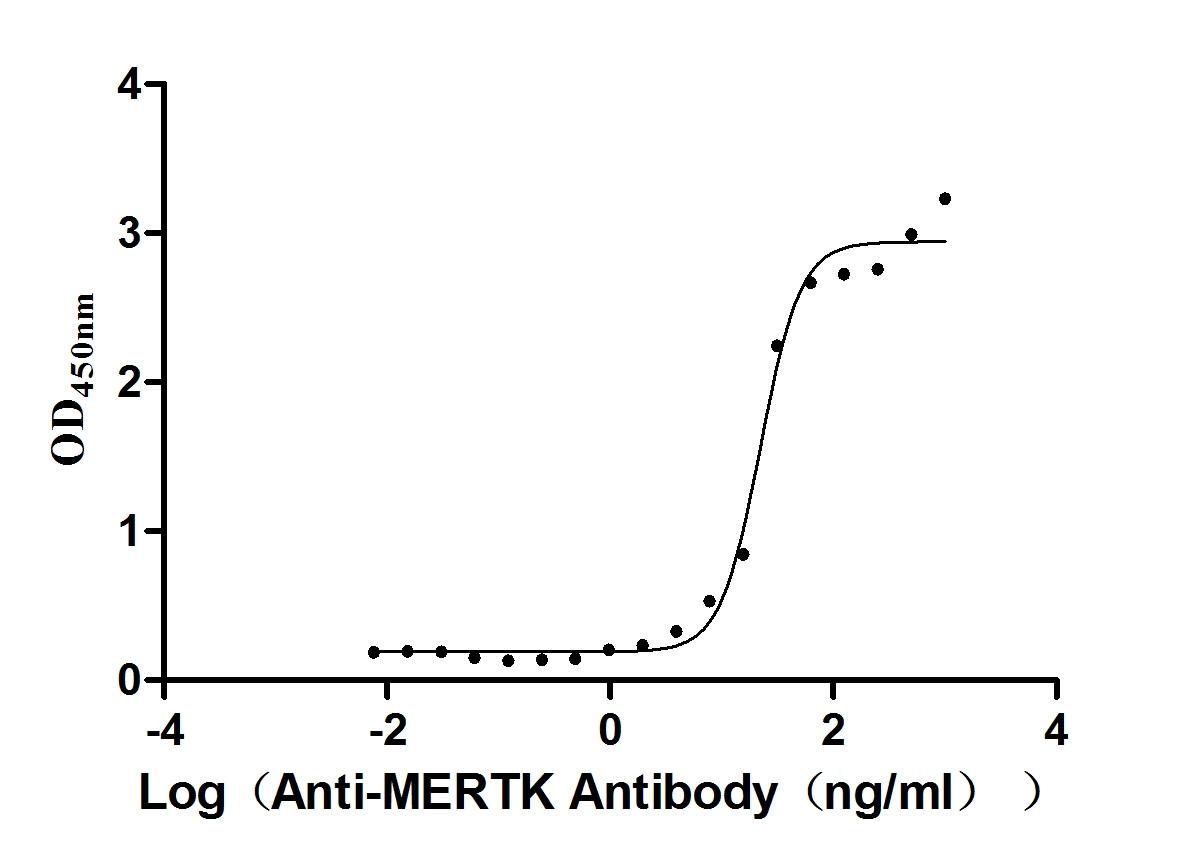
Recombinant Mouse Tyrosine-protein kinase Mer (Mertk), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
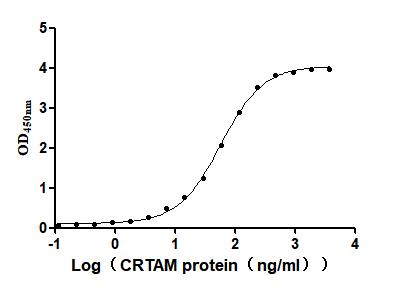
Recombinant Mouse Cytotoxic and regulatory T-cell molecule (Crtam), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
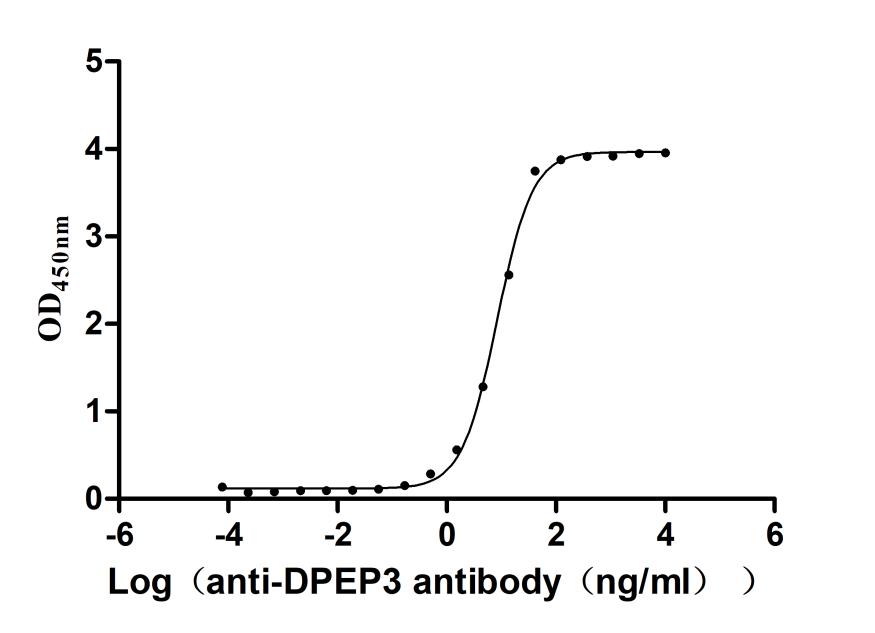
Recombinant Macaca fascicularis Dipeptidase 3(DPEP3) (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Express system: Mammalian cell
Species: Homo sapiens (Human)