Recombinant Escherichia coli Multidrug transporter emrE (emrE), partial
-
中文名称:大肠杆菌emrE重组蛋白
-
货号:CSB-YP340580ENV1
-
规格:
-
来源:Yeast
-
其他:
-
中文名称:大肠杆菌emrE重组蛋白
-
货号:CSB-EP340580ENV1
-
规格:
-
来源:E.coli
-
其他:
-
中文名称:大肠杆菌emrE重组蛋白
-
货号:CSB-EP340580ENV1-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名称:大肠杆菌emrE重组蛋白
-
货号:CSB-BP340580ENV1
-
规格:
-
来源:Baculovirus
-
其他:
-
中文名称:大肠杆菌emrE重组蛋白
-
货号:CSB-MP340580ENV1
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:emrE
-
Uniprot No.:
-
别名:emrE; eb; mvrC; b0543; JW0531; Multidrug transporter EmrE; Efflux-multidrug resistance protein EmrE; Ethidium resistance protein; Methyl viologen resistance protein C
-
种属:Escherichia coli (strain K12)
-
蛋白长度:Partial
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Multidrug efflux protein that confers resistance to a wide range of toxic compounds, including ethidium, methyl viologen, acriflavine, tetraphenylphosphonium (TPP(+)), benzalkonium, propidium, dequalinium and the aminoglycoside antibiotics streptomycin and tobramycin. Can also transport the osmoprotectants betaine and choline. The drug efflux is coupled to an influx of protons. Can couple antiport of a drug to either one or two protons, performing both electrogenic and electroneutral transport of a single substrate. Simultaneously binds and cotransports proton and drug.
-
基因功能参考文献:
- Optimal positions of the peptide staple were determined using free-energy simulations of peptide binding to monomeric EmrE Three of the four top-scoring peptides selected for experimental testing resulted in significant inhibition of proton-driven ethidium efflux in live cells without nonspecific toxicity. The approach described here is expected to be of general use for the design of peptide therapeutics. PMID: 30082384
- Not only can EmrE export diverse drug substrates, it can couple antiport of a drug to either one or two protons, performing both electrogenic and electroneutral transport of a single substrate. PMID: 29114048
- In a practical application of the BLaTM system, the s find that the antiparallel interaction of TMD4, the known dimerization domain of the dual-topology small multidrug transporter EmrE, is sequence-specific and much stronger than the parallel one. PMID: 28432015
- This work reveals a mechanism for regulating membrane-protein topogenesis, in which initially misintegrated configurations of the proteins undergo post-translational annealing to reach fully integrated multispanning topologies. PMID: 26408961
- The results indicate that a binding pattern can be formed in the EmrE in such a way that GLU14 binds to the positively charged fragment of a substrate molecule, and other aromatic residues. PMID: 26014489
- Based on these structural results, EmrE mutants were created to ascertain whether a specific loop length and composition were necessary for function. PMID: 25406320
- Replacement of the corresponding alanine residue with serine in two different homologues of EmrE enables them to provide robust resistance to methyl viologen in vivo and to transport methyl viologen as determined in proteoliposome assays. PMID: 25479374
- this study has analyzed fused versions of the dual-topology transporter EmrE, a member of the small multidrug resistance family, by blue-native PAGE and in vivo activity measurements. PMID: 24690367
- the rate of interconversion between the inward- and outward-facing states of EmrE varies over 3 orders of magnitude. Thus, the identity of the bound substrate controls the rate of this critical step in the transport process. PMID: 24448799
- The study identified that betaine and choline are natural quaternary cation compounds substrates of EmrE. PMID: 22942246
- Transforming EmrE, a drug/H+ antiporter, into a polyamine importer by a single mutation. PMID: 23035252
- EmrE structure in liposomes and the substrate-induced conformational changes PMID: 20551331
- topology of inner membrane protein EmrE could be controlled by a single positively charged residue placed in different locations throughout the protein, including C terminus; observation points to plasticity in membrane protein insertion mechanisms PMID: 20508091
- EmrE is a proton coupled multi-drug transporter of Escherichia coli PMID: 15189838
- EmrE transports monovalent and divalent substrates with the same stoichiometry PMID: 15371426
- analysis of ligand binding to the multidrug resistance protein EmrE by isothermal titration calorimetry PMID: 15501941
- Crystallography of the integral membrane protein EmrE from Escherichia coli PMID: 15583400
- analysis of a unique carboxyl residue in EmrE using mass spectrometry PMID: 15623511
- Tryptophan 63, the only essential Trp residue in EmrE, is also a sensor for ligand binding and/or conformational changes following the EmrE substrate binding reaction. PMID: 15882076
- analysis of the binding domain of EmrE PMID: 16049002
- topological analysis of EmrE protein PMID: 17003034
- 3-D structure of EmrE acquired by electron cryo-microscopy (cryo-EM) at 7.5 Angstroms resolution in the membrane plane showed that parts of the structure are related by quasi-symmetry PMID: 17005200
- it was experimentally demonstrated that the evolution of the membrane protein, EmrE, composed of two homologous but oppositely oriented domains can occur in a small number of steps PMID: 17255477
- The 3D structures of EmrE have since been retracted because of faulty software, but the suggestion that the protomers in the dimer are in an antiparallel topological orientation sparked controversy that is still ongoing. PMID: 17452106
- Data compare the NMR spectra obtained from wildtype EmrE to those obtained from the mutant EmrE-E14C and the experiments allow to assign the chemical shift of the carboxylic carbon of E14. PMID: 17976529
- study reports the corrected structures as recalculated from the original diffraction, new data from selenomethionine (SeMet)-labeled crystals, and functional assays of the recombinant proteins used for structure determination PMID: 18024586
- Glu14 is a key residue for substrate transport in the EmrE dimer; it is asymmetric PMID: 18042544
- Constraining the relative topology of homodimeric EmrE protomers within the dimer in order to assay EmrE activity in vivo leads to results that support a parallel topology of EmrE protomers in the functional dimer. PMID: 18059473
- Data propose a coupled insertion and assembly model for EmrE in which the favorable energetics of the parallel dimer interface override topological constraints arising from weak asymmetry in positive charge distribution. PMID: 18616286
- The revised X-ray structure with substrate bound suggests that the proposed antiparallel orientation of the monomers is indeed correct; this represents a new structural paradigm in membrane-protein structures. PMID: 19171974
- Results add further weight to the hypothesis that an increased lateral phospholipid chain pressure hinders EmrE insertion across the bilayer. PMID: 19699749
显示更多
收起更多
-
亚细胞定位:Cell inner membrane; Multi-pass membrane protein.
-
蛋白家族:Small multidrug resistance (SMR) protein family
-
数据库链接:
KEGG: ecj:JW0531
STRING: 316385.ECDH10B_0499
Most popular with customers
-
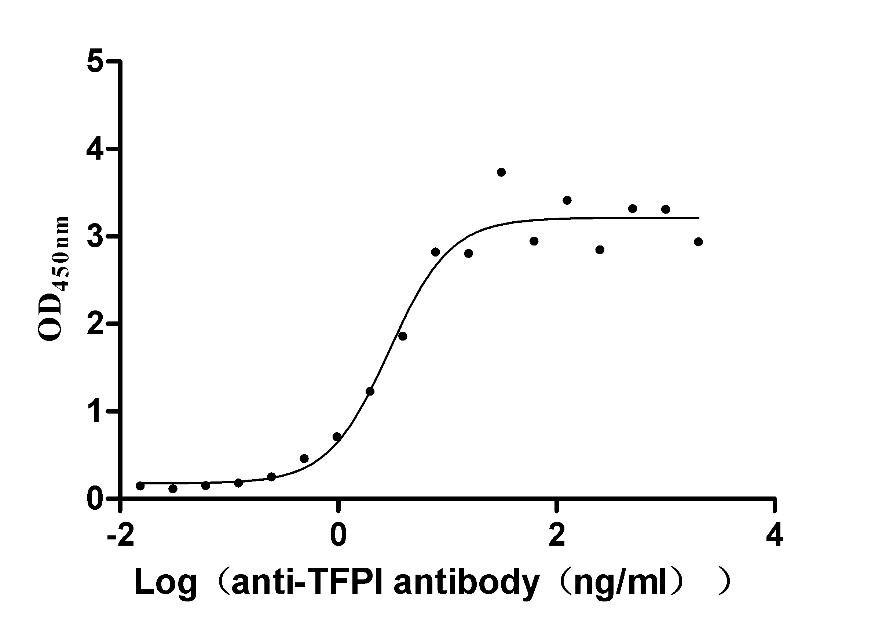
Recombinant Rabbit Tissue factor pathway inhibitor (TFPI) (Active)
Express system: Mammalian cell
Species: Oryctolagus cuniculus (Rabbit)
-
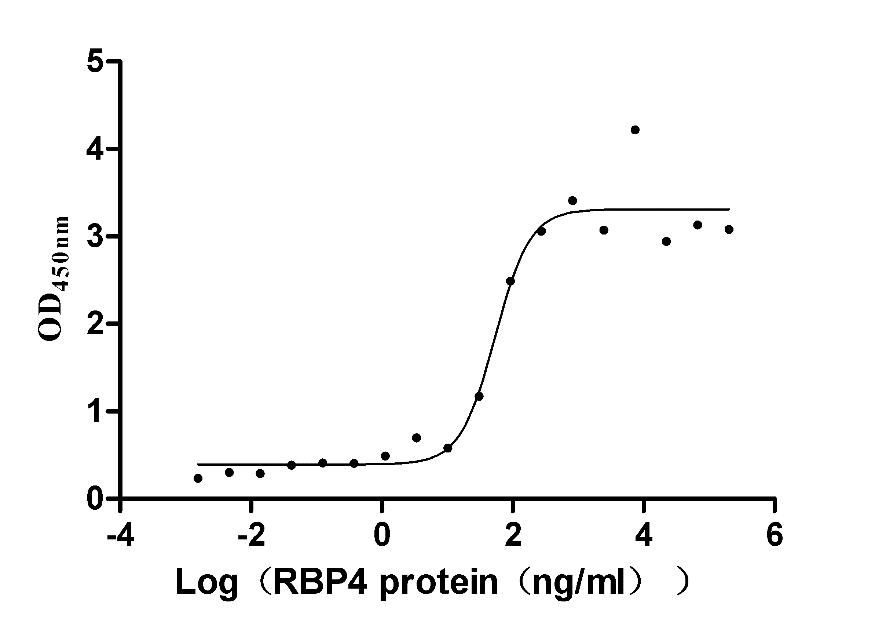
Recombinant Mouse Retinol-binding protein 4 (Rbp4) (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
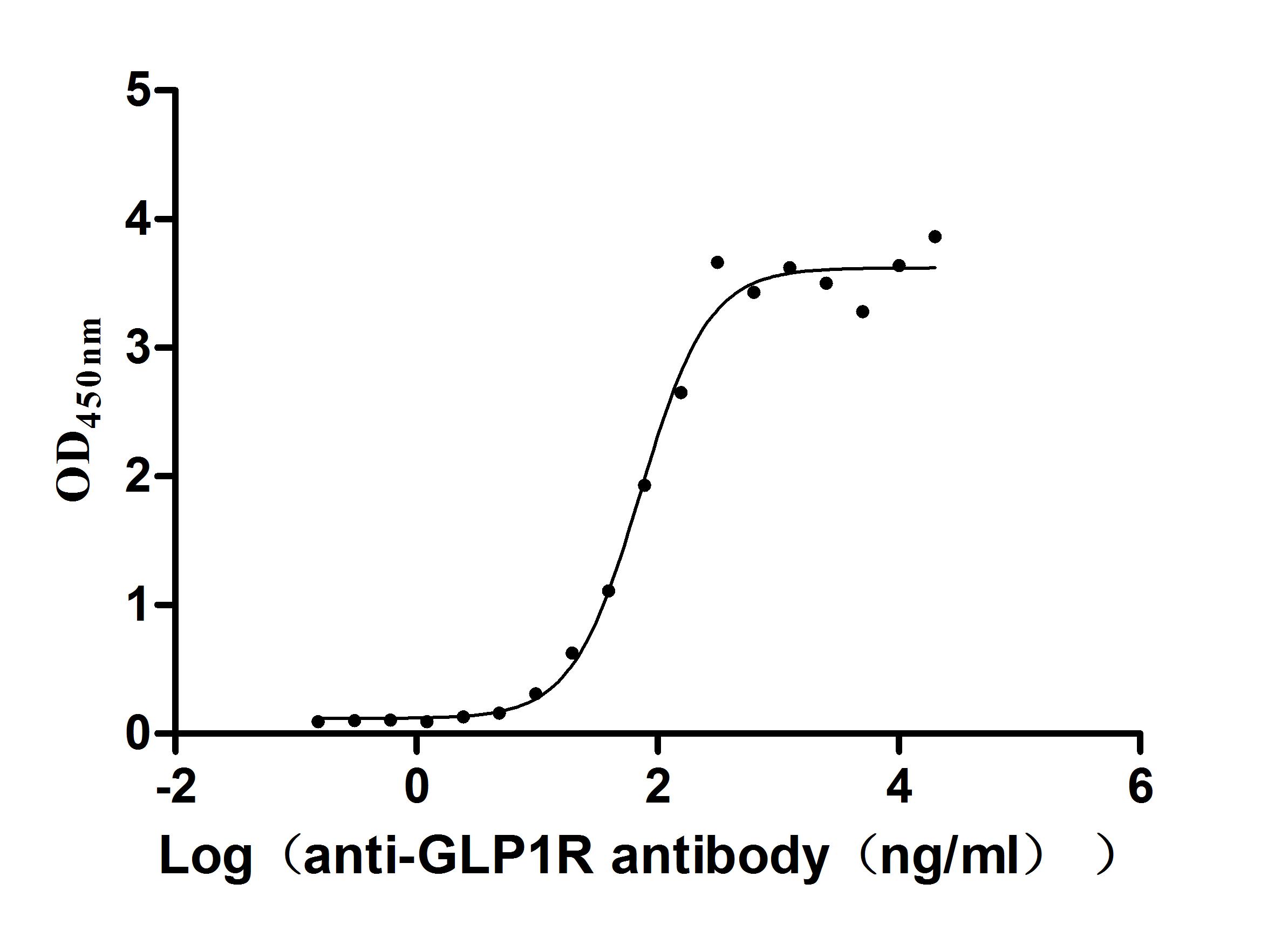
Recombinant Human Glucagon-like peptide 1 receptor (GLP1R), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Dickkopf-related protein 1 (DKK1) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
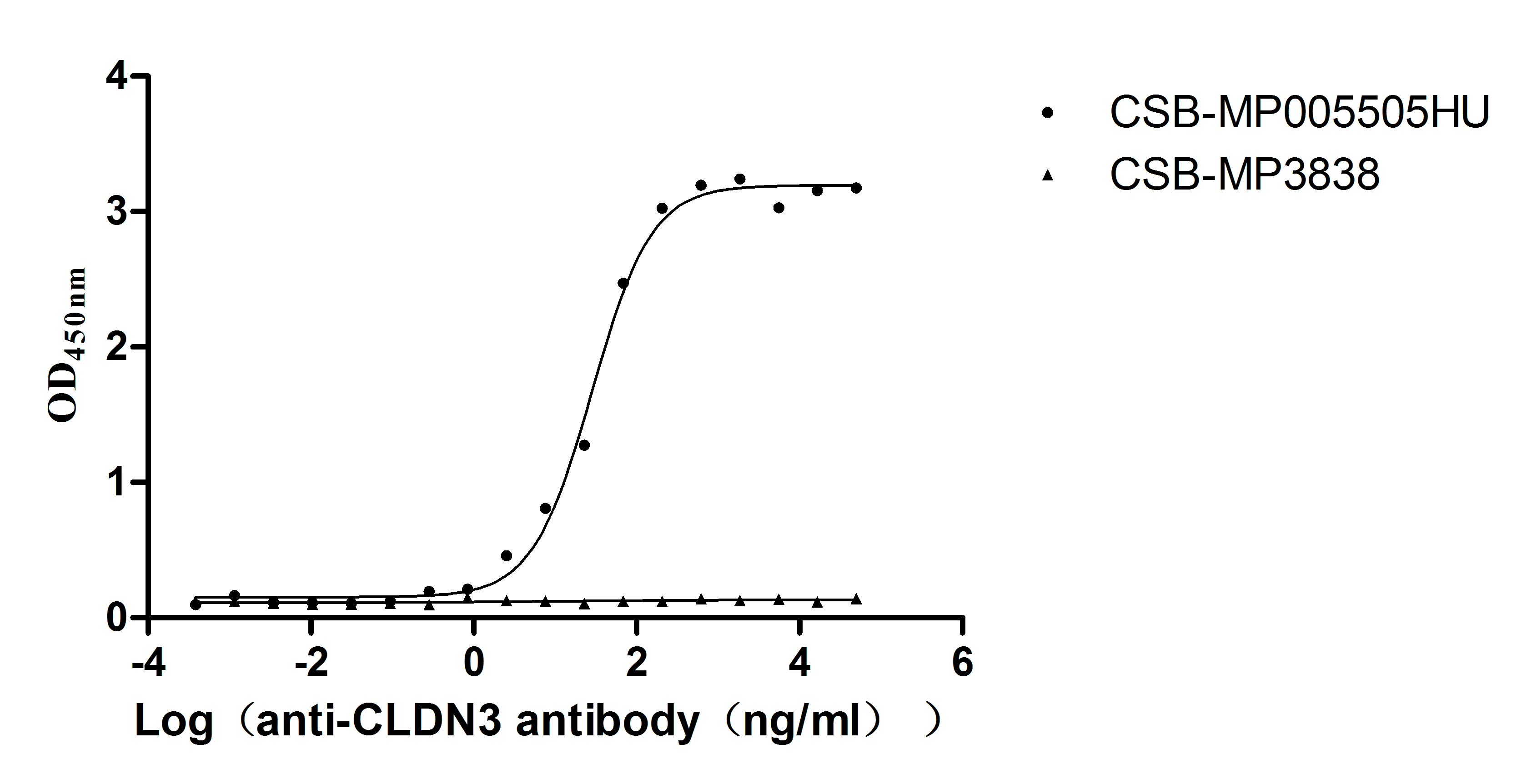
Recombinant Human Claudin-3 (CLDN3)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
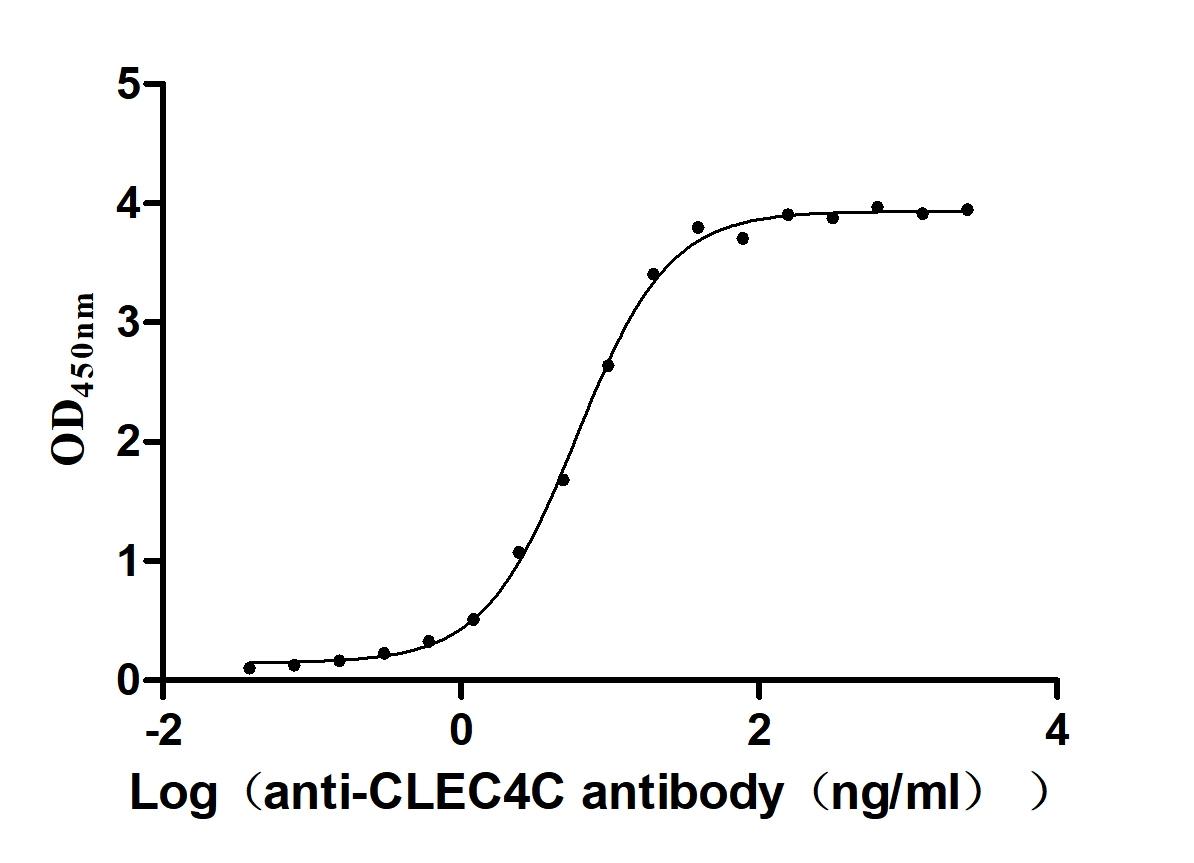
Recombinant Macaca fascicularis C-type lectin domain family 4 member C(CLEC4C), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
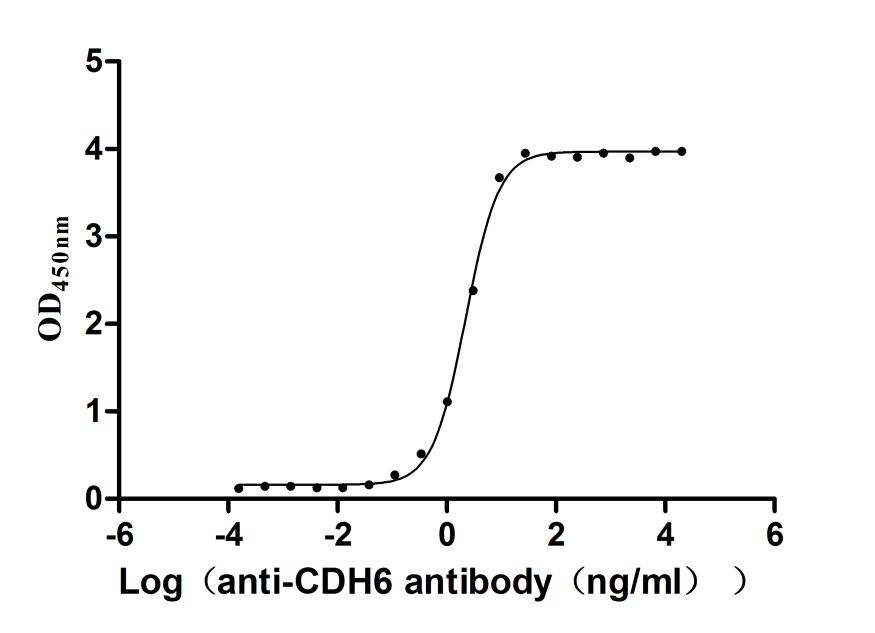
Recombinant Macaca fascicularis Cadherin 6(CDH6),partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
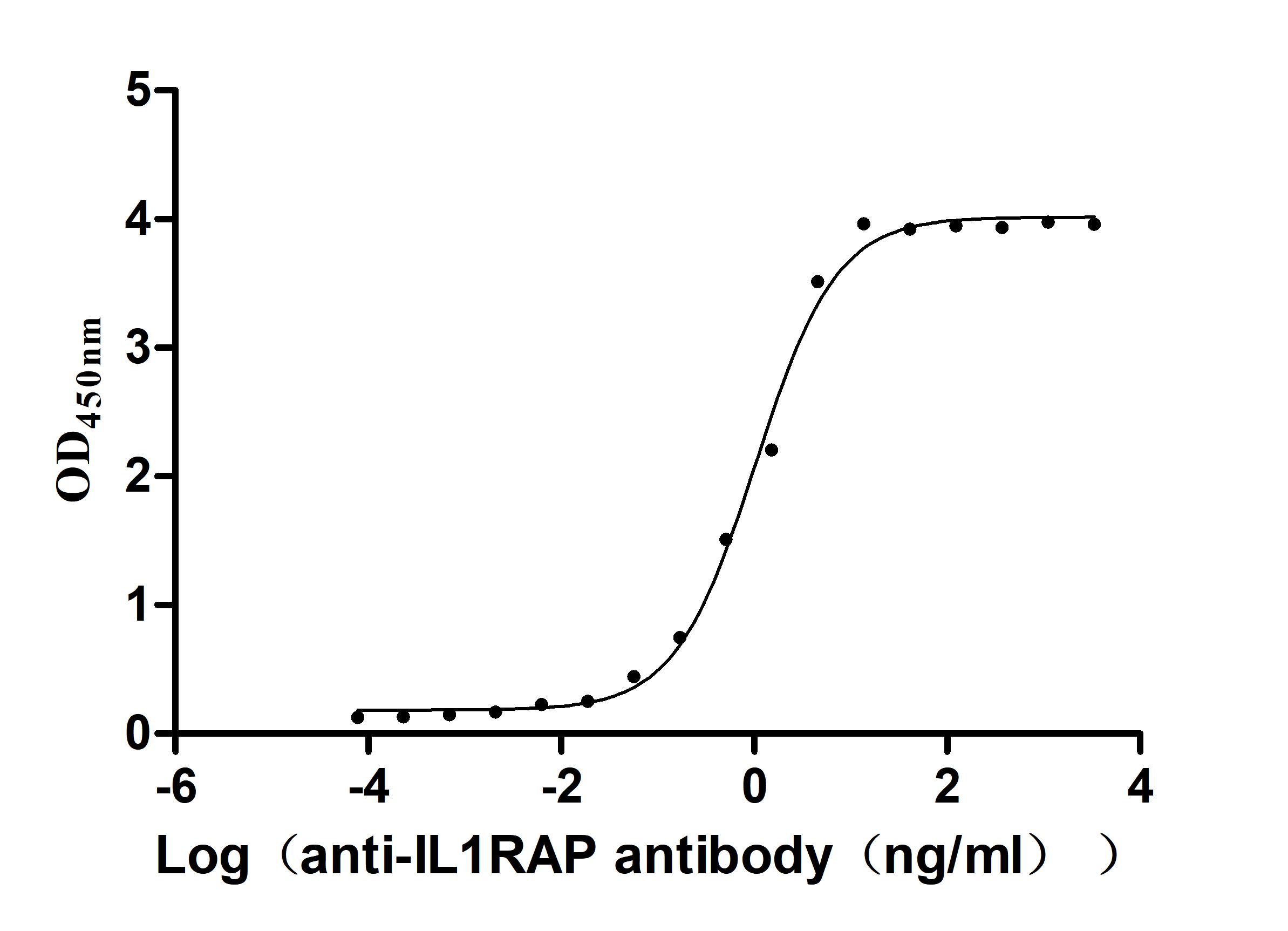
Recombinant Macaca fascicularis Interleukin 1 receptor accessory protein(IL1RAP), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)


-AC1.jpg)