Recombinant Horse Myoglobin (MB)
-
中文名称:Recombinant Horse Myoglobin(MB),Yeast
-
货号:CSB-YP013529HO
-
规格:
-
来源:Yeast
-
其他:
-
中文名称:Recombinant Horse Myoglobin(MB),Yeast
-
货号:CSB-EP013529HO
-
规格:
-
来源:E.coli
-
其他:
-
中文名称:Recombinant Horse Myoglobin(MB),Yeast
-
货号:CSB-EP013529HO-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名称:Recombinant Horse Myoglobin(MB),Yeast
-
货号:CSB-BP013529HO
-
规格:
-
来源:Baculovirus
-
其他:
-
中文名称:Recombinant Horse Myoglobin(MB),Yeast
-
货号:CSB-MP013529HO
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:MB
-
Uniprot No.:
-
别名:MB; Myoglobin
-
种属:Equus caballus (Horse)
-
蛋白长度:Full Length of Mature Protein
-
表达区域:2-154
-
氨基酸序列GLSDGEWQQ VLNVWGKVEA DIAGHGQEVL IRLFTGHPET LEKFDKFKHL KTEAEMKASE DLKKHGTVVL TALGGILKKK GHHEAELKPL AQSHATKHKI PIKYLEFISD AIIHVLHSKH PGDFGADAQG AMTKALELFR NDIAAKYKEL GFQG
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
靶点详情
-
功能:Serves as a reserve supply of oxygen and facilitates the movement of oxygen within muscles.
-
基因功能参考文献:
- A spectroscopic study involving apomyoglobin (Apo-Mb) and cyclodextrin (CyD) of various cavity sizes as model globular protein and synthetic receptors, respectively, using steady-state and picosecond-resolved techniques, is detailed here. PMID: 24089364
- Determination of a representative formal redox potentials of the Fe(II)/Fe(III) redox couple cyanhaemoglobin/cyanmethaemoglobin and the myoglobin/metmyoglobin , at pH=7 and related to the state in solution, was the objective of this work. PMID: 29197256
- Methylglyoxal interacts with myoglobin by Maillard reaction. Methylglyoxal-modification leads to amyloid-like aggregation of Mb at longer incubation time. PMID: 26554310
- Results suggest that the regions of apomyoglobin that resist denaturant perturbations form during the earlier stages of folding. PMID: 26244984
- The study applies hydrogen/deuterium exchange (HDX) mass spectrometry (MS) for probing the conformational dynamics of the model protein myoglobin (Mb) in the presence of N(2) bubbles. PMID: 25761782
- Using a coarse-grained symmetrized Go model, study performed a series of folding simulations of two apo-myoglobin molecules restrained at a high density, addressing competition of formation of a domain-swapped dimer with folding to two monomer structures. PMID: 25591933
- This work presents a thorough investigation of the hydration dependence of myoglobin dynamics. PMID: 24309207
- Equine carbonmonoxy Mb contains 4.5 +/- 1.0 ordered internal water molecules with a mean survival time of 5.6 +/- 0.5 mus at 25 degrees C. PMID: 24195787
- this report presents a series of time-resolved UV/vis spectroscopy experiments in which no ferrylMb was detected when oxyMb and NO reacted. PMID: 23768169
- The stretching mode of nitric oxide (NO) in ferric MB(III)NO consists of a major band at 1922 cm(-1) (97.7%) and a minor band at 1902 cm(-1) (2.3%), suggesting that ferric MB(III)NO in room temperature solution has two conformational substates. PMID: 23432208
- The study computes electron transfer rates for the [myoglobin(wt), cytochrome b5] complex. PMID: 22955681
- The ultrafast equilibrium fluctuations of the Fe(III)-NO complex of a single point mutation of Myoglobin (H64Q) have been studied using Fourier Transform 2D-IR spectroscopy. PMID: 22526234
- Various structural models for the first stable HNO heme protein complex, MbHNO (Mb, myoglobin), were examined by quantum chemical calculations. PMID: 21834502
- Results describe a correlation between glycation-induced structural and functional modifications of the heme protein myoglobin. PMID: 20091095
- Electrospray ionization mass spectrometry (ESI-MS) was employed to monitor the heme release and the conformational changes of myoglobin (Mb) under different solvent conditions, and to observe ligand bindings of Mb. PMID: 20527030
- The result indicates that the coupled protein motions involve collective motions extended over entire myoglobin correlated with local gating motions at the channels. PMID: 20409486
- Results describe the crystal structure of peroxymyoglobin generated through cryoradiolytic reduction of myoglobin compound III. PMID: 18215120
- Similar conclusions are obtained both for pig cyano-myoglobin and for horse cyano-myoglobin, the structural deformation being in the former of minor entity PMID: 19368018
显示更多
收起更多
-
蛋白家族:Globin family
-
数据库链接:
KEGG: ecb:100054434
STRING: 9796.ENSECAP00000015509
UniGene: Eca.16922
Most popular with customers
-
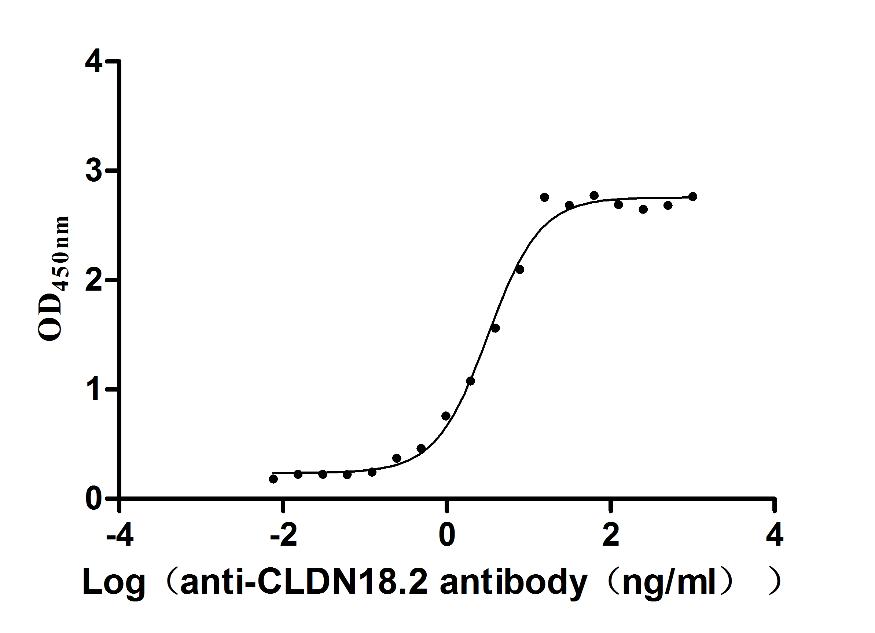
Recombinant Macaca fascicularis Claudin (CLDN18)-VLPs (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Human Desmoglein-3 (DSG3), partial (Active)
Express system: Baculovirus
Species: Homo sapiens (Human)
-
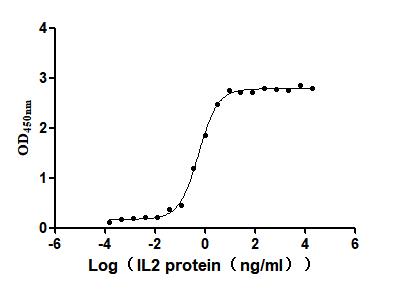
Recombinant Human Interleukin-2 (IL2) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Carcinoembryonic antigen-related cell adhesion molecule 8(CEACAM8) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
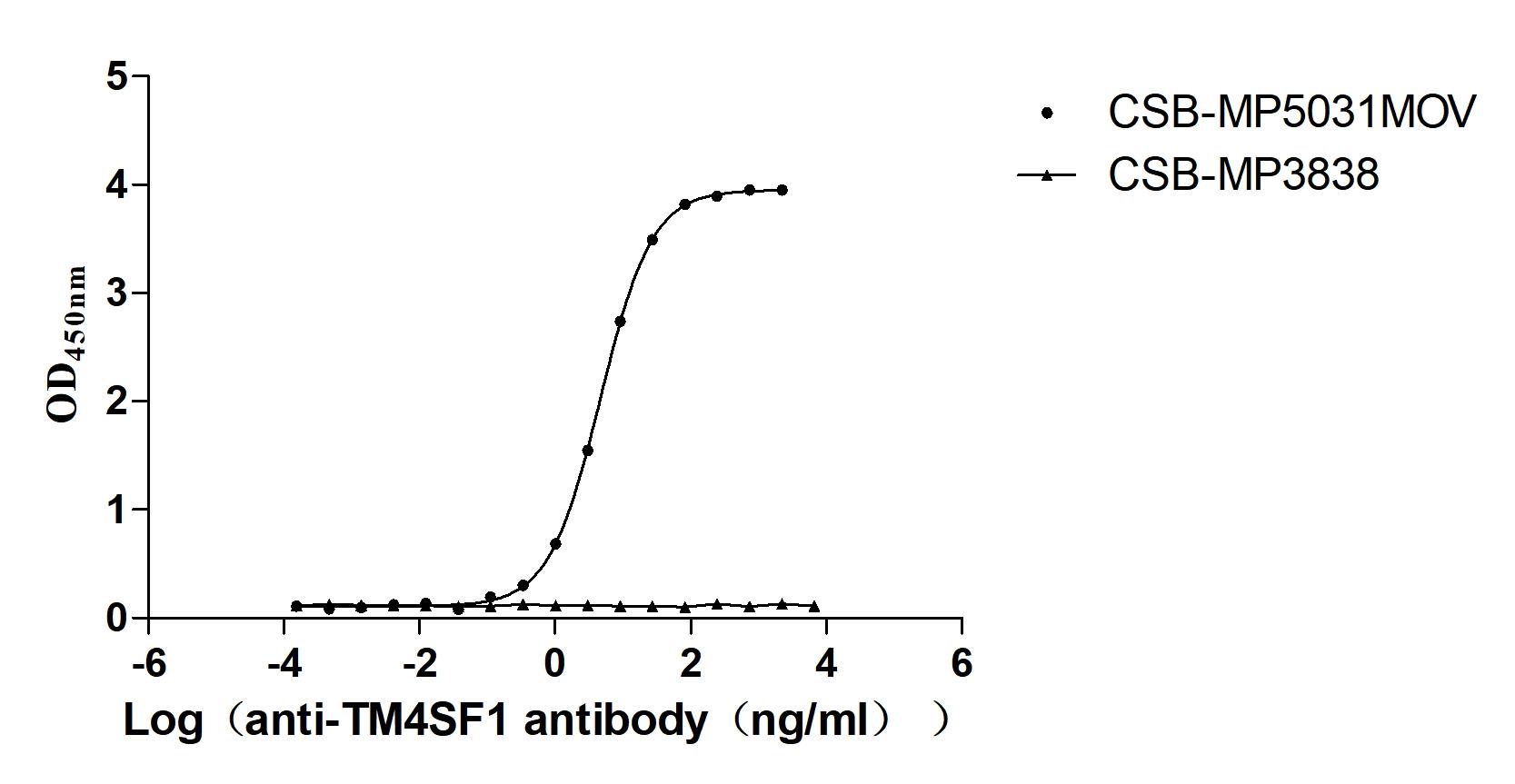
Recombinant Macaca fascicularis Transmembrane 4 L6 family member 1 (TM4SF1)-VLPs (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
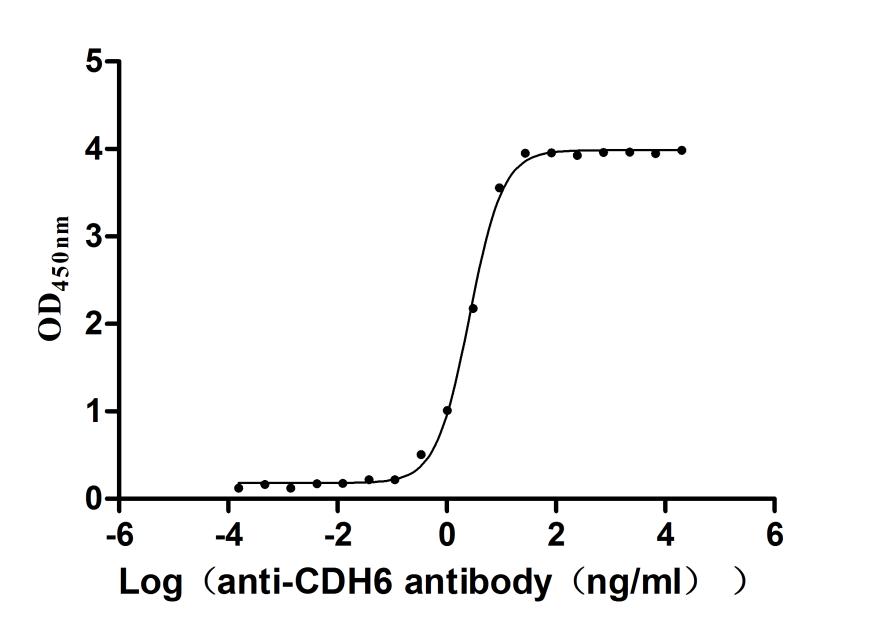
Recombinant Human Cadherin-6(CDH6),partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Cadherin-1(CDH1),partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca fascicularis Dipeptidase 3(DPEP3) (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)