Recombinant Human Krev interaction trapped protein 1 (KRIT1)
-
货号:CSB-YP012501HU
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP012501HU
-
规格:
-
来源:E.coli
-
其他:
-
货号:CSB-EP012501HU-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP012501HU
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP012501HU
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
别名:Ankyrin repeat containing protein Krit1; CAM; CCM 1; CCM1; Cerebral cavernous malformations 1; Cerebral cavernous malformations 1 protein; Krev interaction trapped 1; Krev interaction trapped protein 1; KRIT 1; KRIT1 ankyrin repeat containing; KRIT1; KRIT1_HUMAN
-
种属:Homo sapiens (Human)
-
蛋白长度:Full length protein
-
表达区域:1-736
-
氨基酸序列MGNPENIEDA YVAVIRPKNT ASLNSREYRA KSYEILLHEV PIEGQKKKRK KVLLETKLQG NSEITQGILD YVVETTKPIS PANQGIRGKR VVLMKKFPLD GEKMGREASL FIVPSVVKDN TKYTYTPGCP IFYCLQDIMR VCSESSTHFA TLTARMLIAL DKWLDERHAQ SHFIPALFRP SPLERIKTNV INPAYATESG QTENSLHMGY SALEIKSKML ALEKADTCIY NPLFGSDLQY TNRVDKVVIN PYFGLGAPDY SKIQIPKQEK WQRSMSSVTE DKERQWVDDF PLHRSACEGD SELLSRLLSE RFSVNQLDSD HWAPIHYACW YGKVEATRIL LEKGKCNPNL LNGQLSSPLH FAAGGGHAEI VQILLNHPET DRHITDQQGR SPLNICEENK QNNWEEAAKL LKEAINKPYE KVRIYRMDGS YRSVELKHGN NTTVQQIMEG MRLSQETQQY FTIWICSENL SLQLKPYHKP LQHVRDWPEI LAELTNLDPQ RETPQLFLRR DVRLPLEVEK QIEDPLAILI LFDEARYNLL KGFYTAPDAK LITLASLLLQ IVYGNYESKK HKQGFLNEEN LKSIVPVTKL KSKAPHWTNR ILHEYKNLST SEGVSKEMHH LQRMFLQNCW EIPTYGAAFF TGQIFTKASP SNHKVIPVYV GVNIKGLHLL NMETKALLIS LKYGCFMWQL GDTDTCFQIH SMENKMSFIV HTKQAGLVVK LLMKLNGQLM PTERNS
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Component of the CCM signaling pathway which is a crucial regulator of heart and vessel formation and integrity. Negative regulator of angiogenesis. Inhibits endothelial proliferation, apoptosis, migration, lumen formation and sprouting angiogenesis in primary endothelial cells. Promotes AKT phosphorylation in a NOTCH-dependent and independent manner, and inhibits ERK1/2 phosphorylation indirectly through activation of the DELTA-NOTCH cascade. Acts in concert with CDH5 to establish and maintain correct endothelial cell polarity and vascular lumen and these effects are mediated by recruitment and activation of the Par polarity complex and RAP1B. Required for the localization of phosphorylated PRKCZ, PARD3, TIAM1 and RAP1B to the cell junction, and cell junction stabilization. Plays a role in integrin signaling via its interaction with ITGB1BP1; this prevents the interaction between ITGB1 and ITGB1BP1. Microtubule-associated protein that binds to phosphatidylinositol 4,5-bisphosphate (PIP2)-containing membranes in a GTP-bound RAP1-dependent manner. Plays an important role in the maintenance of the intracellular reactive oxygen species (ROS) homeostasis to prevent oxidative cellular damage. Regulates the homeostasis of intracellular ROS through an antioxidant pathway involving FOXO1 and SOD2. Facilitates the down-regulation of cyclin-D1 (CCND1) levels required for cell transition from proliferative growth to quiescence by preventing the accumulation of intracellular ROS through the modulation of FOXO1 and SOD2 levels.
-
基因功能参考文献:
- We report a case of a highly penetrant but variably expressed form of cerebral cavernous malformation syndrome with cerebral, cutaneous, and retinal cavernomas in a family found to harbor a nonsense mutation of the CCM1 gene. PMID: 28867399
- Case-control study to investigate the possible association of others polymorphisms (c.485+65 C/G, c.989+63 C/G, c.1980 A/G in CCM1 gene, c.472+127 C/T in CCM2 and c.150 G/A in CCM3) with cerebral cavernous malformations. The five polymorphisms were characterized in 64 sporadic patients and in 90 healthy controls by ASO-PCR. Results suggest that some polymorphisms in CCM genes could play an important role in the disease. PMID: 28870584
- The finding of this study suggests that the novel nonsense mutation c.1159G>T in CCM1 gene is associated with multiple cerebral cavernous malformations, and that CCM1 haploinsufficiency may be the underlying mechanism of multiple cerebral cavernous malformations. PMID: 26643368
- Adrenal calcifications identified on CT scans are common in patients with fCCM and may be a clinically silent manifestation of disease. PMID: 28318403
- A novel KRIT1 heterozygous nonsense mutation (c.1864C>T) segregated with familial cerebral cavernous malformation in a Chinese family. PMID: 28255959
- A novel heterozygous insertion KRIT1 mutation identified in cerebral cavernous malformation patient. mRNA level of KRIT1 were significantly decreased in FCCM subjects. PMID: 28160210
- A novel nonsense mutation in CCM1 were detected in cerebral cavernous malformations patient. PMID: 28000143
- nuclear-cytoplasmic shuttling of ICAP1 influences both integrin activation and KRIT1 localization, presumably impacting nuclear functions of KRIT1. PMID: 28003363
- New Krit1 mutations segregated with cerebral cavernous malformation in Chinese families. PMID: 27649701
- Studies suggest that the 3 proteins of the Cerebral Cavernous Malformations (CCM) complex KRIT1/CCM1, CCM2/malcavernin and CCM3/PDCD10 not only require one another for reciprocal stabilization, but also act as a platform for signal transduction. PMID: 26356566
- Valproic acid reduces intracellular ROS level by the modulation of KRIT1 and its correlated proteins, FoxO1, SOD2, and cyclin D1 in mesenchymal stromal cells. PMID: 25203678
- KRIT1 protects endothelial integrity during mechanical stress and trap6 exposure. PMID: 25923142
- Novel CCM1 deletion mutation segregated with cerebral cavernous angioma in a Chinese family. PMID: 25185960
- Case Report: cerebral cavernous malformations and unilateral moyamoya disease in a patient with a new mutation in the KRIT-1 /CCM1 gene. PMID: 25413039
- Here we discuss nuclear functions of adhesion complex proteins with a special focus on the CCM-1/KRIT-1 protein, which may turn out to be yet another adhesion complex protein with a second life. PMID: 24714220
- Data find that several disease-associated missense mutations in CCM2 have the potential to interrupt the KRIT1-CCM2 interaction by destabilizing the CCM2 PTB domain and that a KRIT1 mutation also disrupts this interaction PMID: 25525273
- Genetic analysis of familial cerebral cavernous malformation in Japanese involved the KRIT1 gene. PMID: 25707093
- Data indicate the regulatioin of vascular endothelial growth factor (VEGF) signaling in Krev-interaction trapped 1 (KRIT1)-depleted endothelial cells. PMID: 25320085
- Data indicate that the major binding site for binding of sorting nexin 17 (SNX17) is confined to the NPXF2 motif in cytoplasmic adaptor protein Krev interaction trapped 1 (KRIT1). PMID: 25059659
- Our findings demonstrate that CCM1 c.263-10A > G mutation is associated with cerebral cavernous malformations. PMID: 25086949
- Prevalence, frequency and characterization of CCM1, CCM2 and CCM3 variants in cerebral cavernous malformation Spanish patients. PMID: 24466005
- These preliminary data indicate a still undescribed and unknown role for KRIT1 inside the nucleus. PMID: 24705002
- with the reported role of c-Jun in vascular dysfunctions triggered by oxidative stress, our findings shed new light on the molecular mechanisms underlying KRIT1 function and CCM pathogenesis. PMID: 24291398
- DNA sequencing and deletion/duplication testing of the CCM1, CCM2, and CCM3 genes in the proband revealed a CCM1 c.601CNG mutation. PMID: 24007869
- The identification of other four new mutations in 40 sporadic patients with either single or multiple cerebral cavernous malformations, is reported. PMID: 24058906
- A high resolution crystal structure and minimal binding region with integrin cytoplasmic domain-associated protein-1 (ICAP1) has been determined for KRIT1. PMID: 23695561
- KRIT1 mutations are associated with cerebral cavernous malformation in some Japanese patients. PMID: 23485406
- we present a rare and interesting case of monozygotic twins heterozygous for the CCM1 germline mutation and discuss their similar age and type of disease manifestation and their beginning divergent clinical course PMID: 23584803
- These data show that HEG1 can recruit the Rap1-KRIT complex to the plasma membrane. PMID: 23814056
- Our findings suggest that miR-21 promotes tumor cell growth, at least in part, by down-modulating the potential tumor suppressor KRIT1. PMID: 23872064
- Genetic testing identified a truncating mutation in the KRIT1 gene in one individual and confirmed the diagnosis of familial cerebral cavernomas as the cause of epilepsy in a family. PMID: 22699465
- The structural basis for KRIT1 antagonized ICAP1-modulated integrin-beta1 activation. PMID: 23317506
- Structural basis for small G protein effector interaction of Ras-related protein 1 (Rap1) and adaptor protein Krev interaction trapped 1 (KRIT1) PMID: 22577140
- These studies establish that the direct physical interaction of Rap1 with KRIT1 enables the translocation of microtubule-sequestered KRIT1 to junctions, thereby supporting junctional integrity and cardiovascular development PMID: 21633110
- Among familial cases of Cerebral cavernous malformations 67% had a mutation in CCM1, 5.5% in CCM2, and 5.5% in CCM3 PMID: 21029238
- Genetic variations could interfere with the proper CCM1/CCM2/CCM3 protein complex, thus explaining the observed clinical variability in cerebral cavernous malformations in a large family. PMID: 20419355
- Here we report a novel deletion of CCM1 that correlates strongly with cerebral cavernous malformations in a Chinese family. We predict this deletion produces a premature stop code (TGA) at NT 1228, resulting in a truncated protein of 409 amino acids. PMID: 20884211
- We described the complete loss of 7q21.2 locus encompassing the KRIT1 gene and 4 flanking genes in a CCM family by using a dense set of 12 microsatellite markers. PMID: 20798775
- Frame shift mutation in KRIT1 gene is associated with cerebral and multiple spinal cavernous malformations. PMID: 20689144
- We have identified a novel mutation in the KRIT1/CCM1 gene in a patient with both CCM and retinal cavernous hemangioma. PMID: 20306072
- The KRIT1-CCM2 interaction regulates endothelial junctional stability and vascular barrier function by suppressing activation of the RhoA/ROCK signaling pathway. This pathway is dysregulated in human cerebral cavernous malformation endothelium. PMID: 20308363
- Familial CCM1 cavernous malformations are unlikely to be associated with developmental venous anomalies, and sporadic malformations have a high rate of association with them PMID: 19833796
- KRIT1/CCM1 mutation in a patient with retinal and cerebral cavernous angiomas. PMID: 11831930
- Integrin-binding protein also interacts with Krit 1 protein, cause of CCM1. (ICAP-1, integrin cytoplasmic domain-associated protein 1) PMID: 11854171
- In familial CCM1 samples, two new Krit1 mutations have been reported in four new upstream exons and six additional mutations have been discovered in the previously identified exons, demonstrating a mutation frequency of 47% in the 27 families studied. PMID: 11914398
- RNA analysis reveals that two point mutations actually activate cryptic splice-donor sites, causing aberrant splicing and leading to a frameshift and protein truncation PMID: 11941540
- Krit1 mRNA expression in adult tissues PMID: 12204286
- Mutations in Krit1 are associated with familial cerebral cavernous malformations, but seldom a cause of sporadic cavernomas PMID: 12682320
- Identification of a novel KRIT1 mutation in an Italian family with cerebral cavernous malformation by the protein truncation test. PMID: 12810002
- We report a mutation causing a premature termination triplet in exon 17 of the Krit1 gene in a family with diagnoses of cerebral cavernous angioma PMID: 12877753
显示更多
收起更多
-
相关疾病:Cerebral cavernous malformations 1 (CCM1)
-
亚细胞定位:Cytoplasm, cytoskeleton. Cell membrane; Peripheral membrane protein. Cell junction. Note=KRIT1 and CDH5 reciprocally regulate their localization to endothelial cell-cell junctions. Association with RAP1 relocalizes KRIT1 from microtubules to cell junction membranes. Translocates from the cytoplasm along microtubules to the cell membrane in a ITGB1BP1-dependent manner.
-
组织特异性:Low levels in brain. Very weak expression found in heart and muscle.
-
数据库链接:
HGNC: 1573
OMIM: 116860
KEGG: hsa:889
STRING: 9606.ENSP00000344668
UniGene: Hs.531987
Most popular with customers
-
Recombinant Human Poliovirus receptor (PVR) (I340M), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
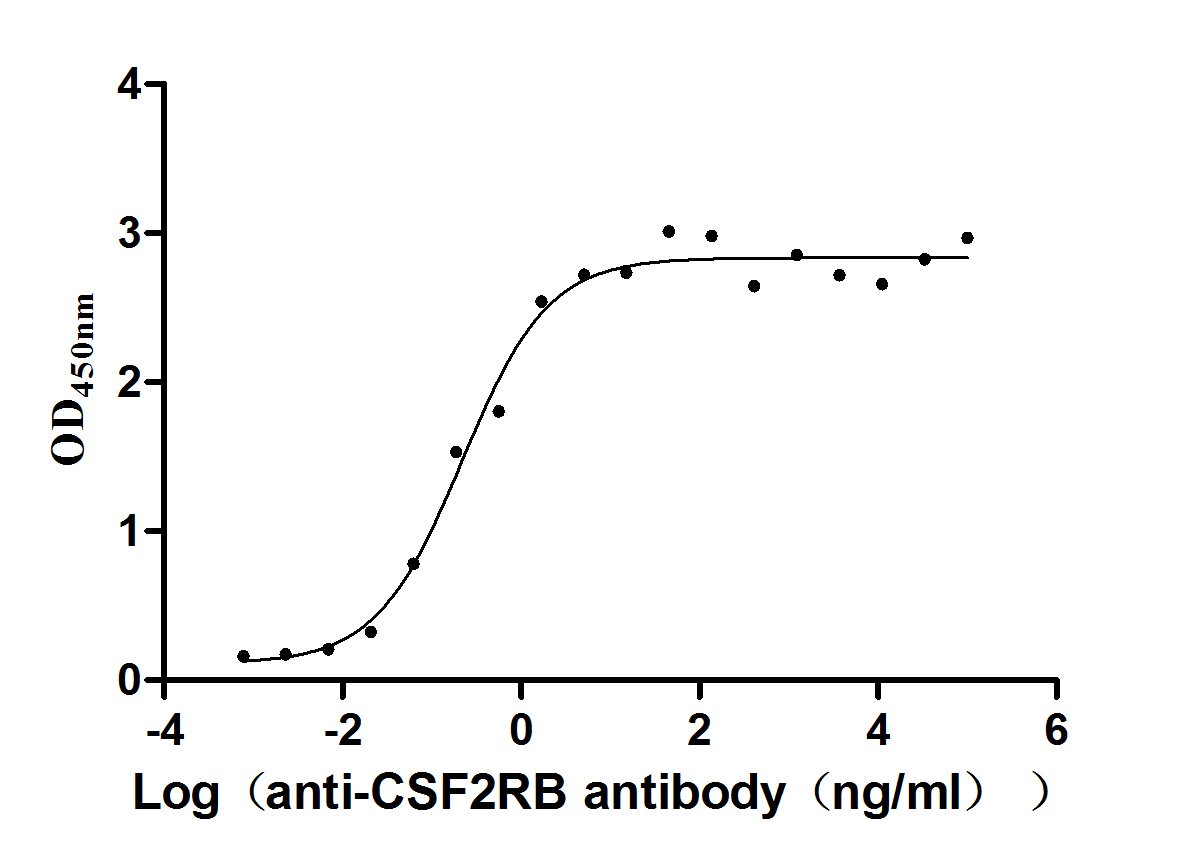
Recombinant Human Cytokine receptor common subunit beta (CSF2RB), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
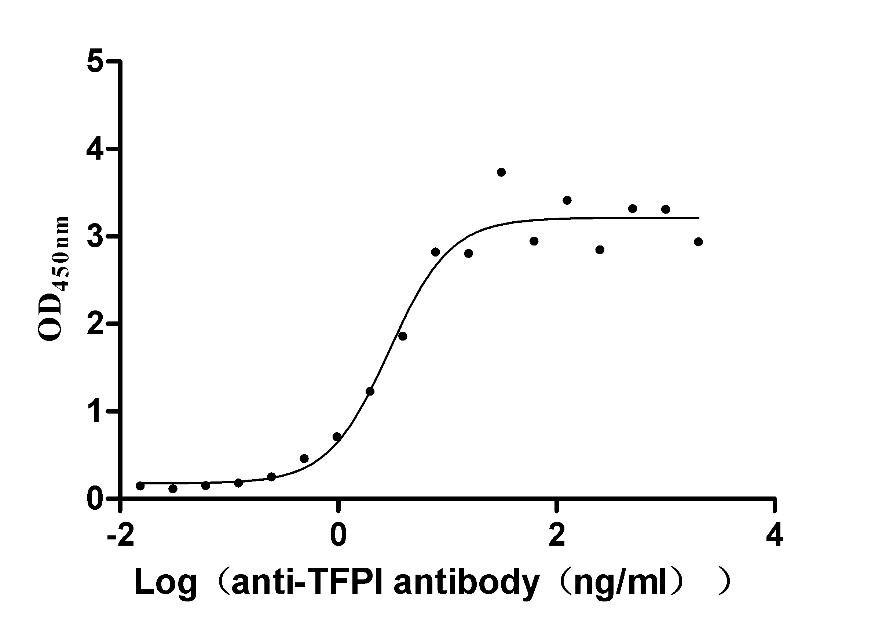
Recombinant Rabbit Tissue factor pathway inhibitor (TFPI) (Active)
Express system: Mammalian cell
Species: Oryctolagus cuniculus (Rabbit)
-
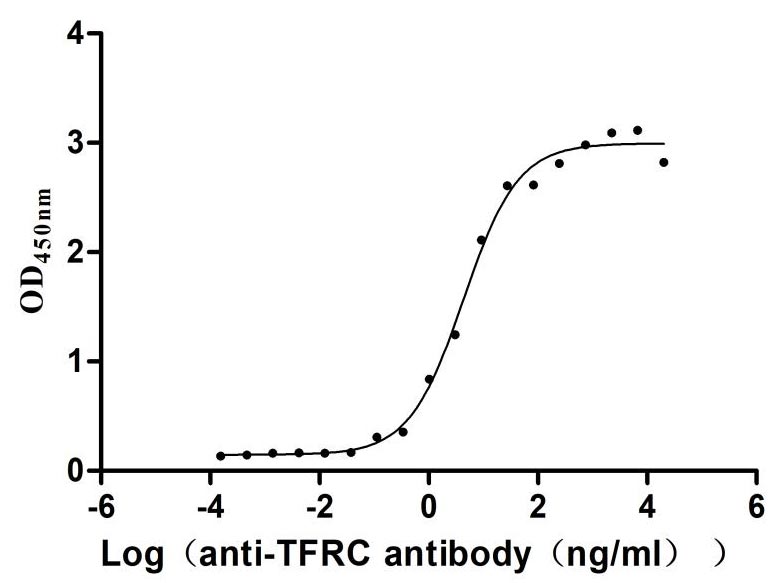
Recombinant Human Transferrin receptor protein 1 (TFRC), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
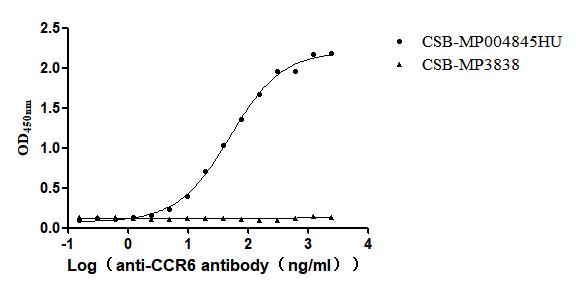
Recombinant Human C-C chemokine receptor type 6(CCR6)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Kidney-associated antigen 1(KAAG1) (Active)
Express system: Baculovirus
Species: Homo sapiens (Human)
-
Recombinant Human Urokinase-type plasminogen activator(PLAU) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Tyrosine-protein kinase receptor UFO(AXL),partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)


-AC1.jpg)