Recombinant Human Non-homologous end-joining factor 1 (NHEJ1)
-
中文名称:人NHEJ1重组蛋白
-
货号:CSB-YP881005HU
-
规格:
-
来源:Yeast
-
其他:
-
中文名称:人NHEJ1重组蛋白
-
货号:CSB-EP881005HU
-
规格:
-
来源:E.coli
-
其他:
-
中文名称:人NHEJ1重组蛋白
-
货号:CSB-EP881005HU-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名称:人NHEJ1重组蛋白
-
货号:CSB-BP881005HU
-
规格:
-
来源:Baculovirus
-
其他:
-
中文名称:人NHEJ1重组蛋白
-
货号:CSB-MP881005HU
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:NHEJ1
-
Uniprot No.:
-
别名:Cernunnos; FLJ12610; NHEJ 1; Nhej1; NHEJ1, S. cerevisiae, homolog of; NHEJ1_HUMAN; Non homologous end joining factor 1; Non-homologous end-joining factor 1; Nonhomologous end joining factor 1; OTTHUMP00000164168; OTTHUMP00000206275; OTTHUMP00000206279; Protein cernunnos; XLF; XRCC4 like factor; XRCC4-like factor
-
种属:Homo sapiens (Human)
-
蛋白长度:full length protein
-
表达区域:1-299
-
氨基酸序列MEELEQGLLM QPWAWLQLAE NSLLAKVFIT KQGYALLVSD LQQVWHEQVD TSVVSQRAKE LNKRLTAPPA AFLCHLDNLL RPLLKDAAHP SEATFSCDCV ADALILRVRS ELSGLPFYWN FHCMLASPSL VSQHLIRPLM GMSLALQCQV RELATLLHMK DLEIQDYQES GATLIRDRLK TEPFEENSFL EQFMIEKLPE ACSIGDGKPF VMNLQDLYMA VTTQEVQVGQ KHQGAGDPHT SNSASLQGID SQCVNQPEQL VSSAPTLSAP EKESTGTSGP LQRPQLSKVK RKKPRGLFS
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:DNA repair protein involved in DNA non-homologous end joining (NHEJ); required for double-strand break (DSB) repair and V(D)J recombination. Plays a key role in NHEJ by promoting the ligation of various mismatched and non-cohesive ends. Together with PAXX, collaborates with DNA polymerase lambda (POLL) to promote joining of non-cohesive DNA ends. May act in concert with XRCC5-XRCC6 (Ku) to stimulate XRCC4-mediated joining of blunt ends and several types of mismatched ends that are non-complementary or partially complementary. Associates with XRCC4 to form alternating helical filaments that bridge DNA and act like a bandage, holding together the broken DNA until it is repaired. The XRCC4-NHEJ1/XLF subcomplex binds to the DNA fragments of a DSB in a highly diffusive manner and robustly bridges two independent DNA molecules, holding the broken DNA fragments in close proximity to one other. The mobility of the bridges ensures that the ends remain accessible for further processing by other repair factors. Binds DNA in a length-dependent manner.
-
基因功能参考文献:
- this study shows loss of NHEJ1 protein due to a novel splice site mutation in a family presenting with combined immunodeficiency, microcephaly, and growth retardation PMID: 28741180
- Phospho-blocking and -mimicking mutations impact both the stability and DNA bridging capacity of XRCC4/XLF complexes, but without affecting their ability to stimulate LIG4 activity. Implicit in this finding is that phosphorylation may regulate DNA bridging by XRCC4/XLF filaments. PMID: 28500754
- Chemotherapy-induced overexpression of XLF and XLF-mediated enhancements in NHEJ activity contribute to chemoresistance in hepatocellular carcinoma (HCC) cells and patients with HCC. Targeting XLF to modulate DSB repair could enhance drug sensitivity and may be a therapeutically useful addition to conventional therapy PMID: 28526069
- Although PAXX-deficient cells lack c-NHEJ phenotypes, PAXX forms a stable ternary complex with Ku bound to DNA. Thus, PAXX plays an accessory role during c-NHEJ that is largely overlapped by XLF's function. PMID: 27705800
- The role of XLF in NHEJ is summarized. PMID: 28846869
- XLF has an important role during V(D)J recombination. PMID: 27281794
- using dual- and quadruple-trap optical tweezers combined with fluorescence microscopy, we show how human XRCC4, XLF and XRCC4-XLF complexes interact with DNA in real time PMID: 27437582
- The data suggest that XLF has multiple functions in DNA repair, and they offer potential explanations for the pleiotropic phenotypes associated with XLF deficiency. PMID: 26100018
- PC4 protects esophageal squamous cell carcinoma cells from IR-induced death by enhancing the nonhomologous end joining-promoting activity of XLF. PMID: 25321468
- Phosphorylation of XLF impairs non-homologous end-joining DNA repair. PMID: 25661488
- Werner syndrome protein positively regulates XRCC4-like factor transcription. PMID: 24626809
- Human XLF is a non-essential, but critical, classic non-homologous end-joining -repair factor. PMID: 24461734
- An induced pluripotent stem cell (iPSC) model of XLF deficiency, which accurately replicates the double-strand break repair deficiency observed in XLF syndrome patients, is reported. PMID: 23818183
- Data indicate that Ku70/Ku80 facilitates the cooperative binding of multiple XRCC4/Ligase IV (XL) and XLF molecules to DNA. PMID: 23620595
- XRCC4 and XLF form long helical protein filaments suitable for DNA end protection and alignment to facilitate DNA double strand break repair. (Review) PMID: 23442139
- Cernunnos deficiency results in chronic activation of the DNA damage response, P53-driven upregulation of proapoptotic factors, leading to decreased thymocyte viability and a qualitative alteration of the T cell repertoire in both humans and mice. PMID: 23207905
- Regulation of Double Strand Break repair by EGFR involves both the NHEJ and HR pathway, and appears to occur in most tumor cell lines regardless of p53 and K-Ras mutation status. PMID: 21665306
- Evidence for how XRCC4-XLF complexes robustly bridge DNA molecules. PMID: 22287571
- A suggested link between defects in the Cernunnos-dependent nonhomologous end-joining pathway and aberrant class switch recombination or switch translocations during the development of B cell malignancies. PMID: 22312109
- Multiple truncations of the XLF and XRCC4 proteins were cocrystallized, but yielded low-resolution diffraction (~20 A) PMID: 22102241
- Data show that XLF and XRCC4 dimers interact through their head domains and form an alternating left-handed helical structure with polypeptide coiled coils and pseudo-dyads of individual XLF and XRCC4 dimers at right angles to the helical axis. PMID: 21936820
- molecular mechanism for XLF-XRCC4 stimulation of DNA ligation. PMID: 21775435
- X-ray structure reveals a filament arrangement for XRCC4(1-157) and Cernunnos(1-224) homodimers mediated by repeated interactions through their N-terminal head domains. PMID: 21768349
- Data show that the heterodimeric domain of Ku was sufficient for the recruitment of XLF to DSBs and for the interaction of Ku with XLF. PMID: 21349273
- NHEJ in human embryonic stem cells is largely independent of ATM, DNA-PKcs, and PARP but dependent on XRCC4 with repair fidelity several-fold greater than in astrocytes. PMID: 20844317
- Patients with NBS-like phenotypes may have mutations in the NHEJ1 gene including multiexon deletions, and show that considerable clinical variability could be observed even within the same family. PMID: 20597108
- identified three amino acids (Arg(64), Leu(65), and Leu(115)) essential for the interaction with X4 and the proper function of Cernunnos PMID: 20558749
- a natural mutator variant of human DNA polymerase lambda promotes chromosomal instability by compromising NHEJ PMID: 19806195
- Cernunnos-XLF is a new Non-homologous End Joining pathway protein, which if mutated results in several conditions -immunodeficiency and developmental anomalies and other conditions, which likely result from inability to repair spontaneous DNA damage. PMID: 16439204
- Studies on the binding of XLF and its role in DNA repair. PMID: 16439205
- Cernunnos physically interacts with the XRCC4 x DNA-ligase IV complex PMID: 16571728
- DNA repair protein involved in lymphocyte activation and cell division. PMID: 16828027
- Results show that a truncated transcript of NHEJ1 is expressed in the polymicrogyric patient cells, suggesting a potential dominant negative effect possibly leading to a different phenotype. PMID: 17191205
- mutant protein retained its ability to stimulate XRCC4.DNA ligase IV but failed to translocate to the nucleus, and this appears to be the basis for the non-homologous DNA end joining defect in this patient PMID: 17317666
- Cernunnos/XRCC4-like factor promotes a mismatched end (MEnd) DNA ligase activity to facilitate joining and to preserve DNA sequence. PMID: 17470781
- Review focuses on the proteins involved in the NHEJ pathway, the major pathway for repair of DNA double-strand breaks, which can become molecular targets in the treatment of cancer. PMID: 17504121
- Data shows that an intact XRCC4/ligase IV complex is necessary for Cernunnos-XLF mobilization to damaged chromatin. PMID: 17720816
- The XLF dimer adopts a similar overall structure to that of the XRCC4 dimer, supporting the contention that these two factors are related in function and have arisen from a common evolutionary ancestor. PMID: 18046455
- Mutational analysis of XLF and XRCC4 reveals a potential interaction interface, suggesting a mechanism for how XLF stimulates the ligation of mismatched ends. PMID: 18158905
- REVIEW: Role and regulation of XLF PMID: 18335491
- The major phosphorylation sites in XLF, serine 245 is phosphorylated by DNA-PK, while serine 251 is phosphorylated by Ataxia-Telangiectasia Mutated (ATM), in vivo. PMID: 18644470
- Results implicate XLF as a C-NHEJ factor but also indicate that developing mouse lymphocytes harbor cell-type-specific factors/pathways that compensate for the absence of XLF function during V(D)J recombination. PMID: 18775323
- the heads and coiled-coil regions of Cernunnos and X4 are not interchangeable, and they suggest specific roles for each in NHEJ PMID: 19103754
- role of Cernunnos/XLF in repair of DSBs and maintenance of genomic stability under replication stress conditions PMID: 19223975
- Recombinant Cernunnos protein restored gap filling and end joining of partially complementary overhangs, and stimulated joining of cohesive ends more than twentyfold. PMID: 19420065
- The XLF-encoded protein (XRRC4 like factor, FLJ12610) is involved in DNA double-strand break repair via nonhomologous end-joining and associates with the Ligase IV-XRCC4 complex. XLF is mutated in a number of radiosensitive and immuno-deficient patients. PMID: 16439205
显示更多
收起更多
-
相关疾病:Severe combined immunodeficiency due to NHEJ1 deficiency (NHEJ1-SCID)
-
亚细胞定位:Nucleus. Chromosome.
-
蛋白家族:XLF family
-
组织特异性:Ubiquitously expressed.
-
数据库链接:
HGNC: 25737
OMIM: 611290
KEGG: hsa:79840
STRING: 9606.ENSP00000349313
UniGene: Hs.225988
Most popular with customers
-
Recombinant Human Angiotensin-converting enzyme 2 (ACE2), partial,Biotinylated (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Signal transducer CD24 (CD24)-Nanoparticle (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Angiopoietin-2 (ANGPT2) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
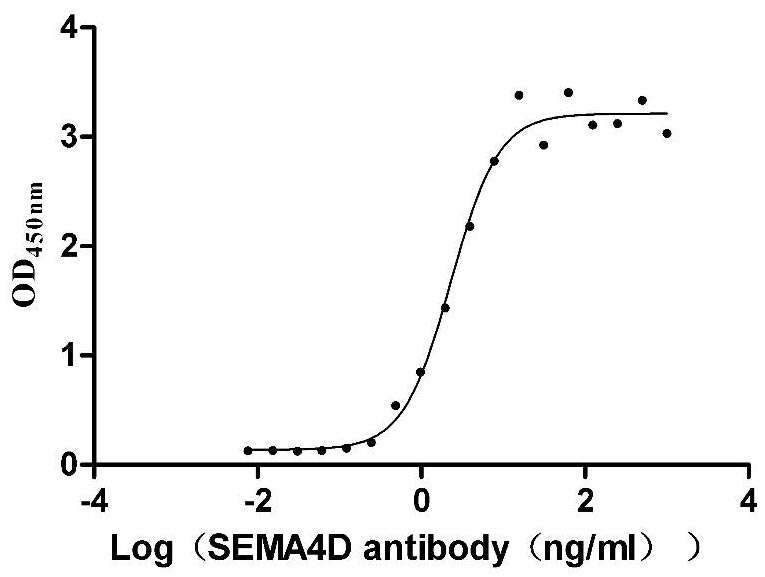
Recombinant Macaca mulatta Semaphorin-4D isoform 1 (SEMA4D), partial (Active)
Express system: Mammalian cell
Species: Macaca mulatta (Rhesus macaque)
-
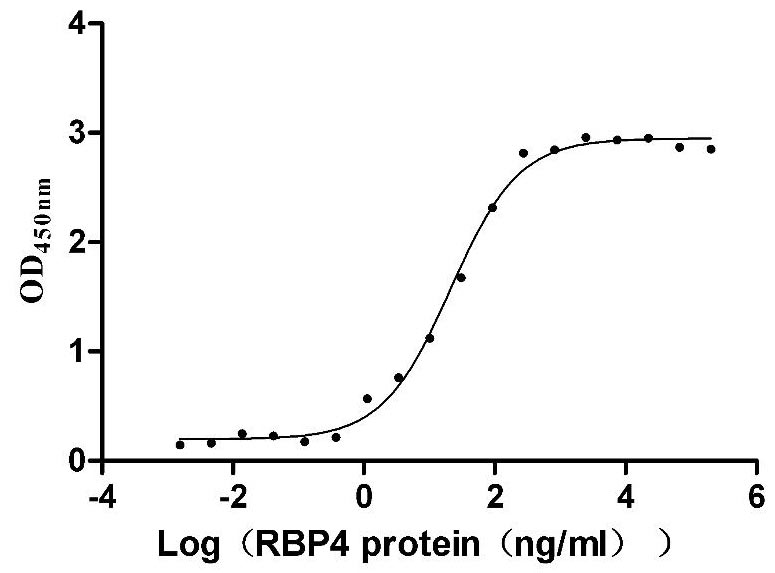
Recombinant Mouse Transthyretin (Ttr) (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
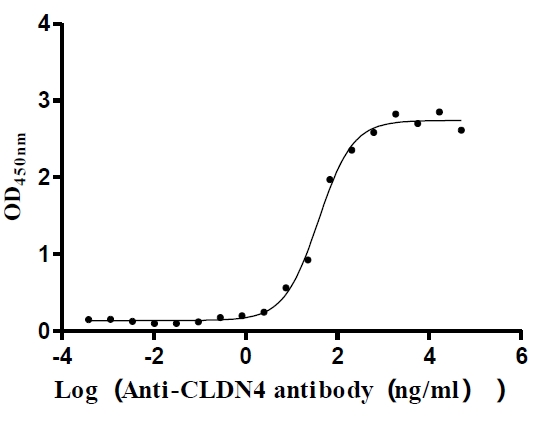
Recombinant Human Claudin-4 (CLDN4)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
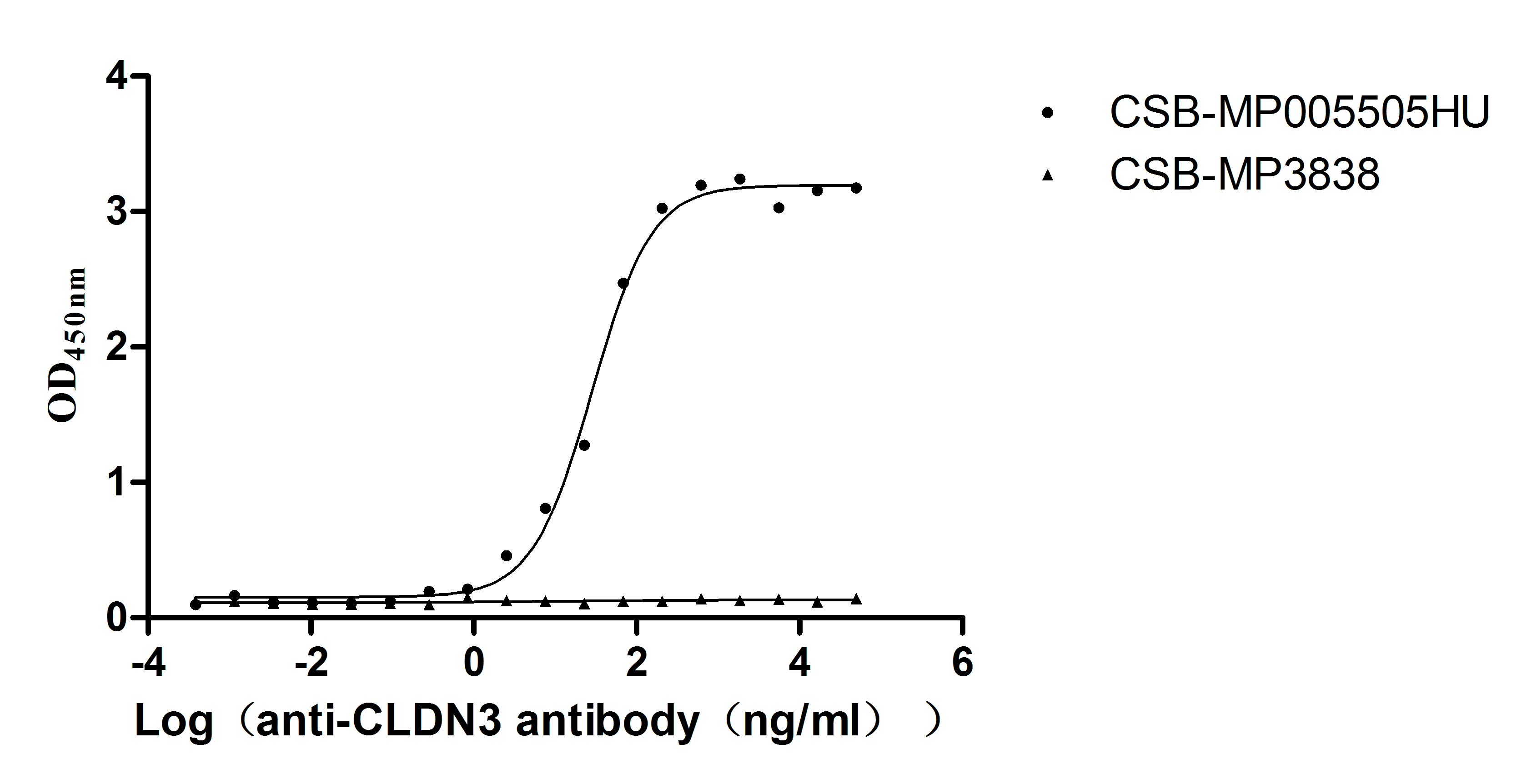
Recombinant Human Claudin-3 (CLDN3)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Transferrin receptor protein 1 (TFRC), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)


-AC1.jpg)