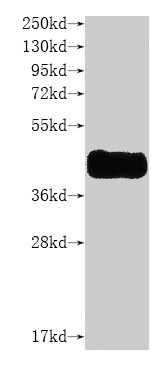
Recombinant Mouse Bcl2 antagonist of cell death (Bad)
-
货号:CSB-YP736816MO
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP736816MO
-
规格:
-
来源:E.coli
-
其他:
-
货号:CSB-EP736816MO-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP736816MO
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP736816MO
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
别名:Bad; Bbc6Bcl2-associated agonist of cell death; BAD; Bcl-2-binding component 6; Bcl-xL/Bcl-2-associated death promoter; Bcl2 antagonist of cell death
-
种属:Mus musculus (Mouse)
-
蛋白长度:full length protein
-
表达区域:1-204
-
氨基酸序列MGTPKQPSLA PAHALGLRKS DPGIRSLGSD AGGRRWRPAA QSMFQIPEFE PSEQEDASAT DRGLGPSLTE DQPGPYLAPG LLGSNIHQQG RAATNSHHGG AGAMETRSRH SSYPAGTEED EGMEEELSPF RGRSRSAPPN LWAAQRYGRE LRRMSDEFEG SFKGLPRPKS AGTATQMRQS AGWTRIIQSW WDRNLGKGGS TPSQ
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Promotes cell death. Successfully competes for the binding to Bcl-X(L), Bcl-2 and Bcl-W, thereby affecting the level of heterodimerization of these proteins with BAX. Can reverse the death repressor activity of Bcl-X(L), but not that of Bcl-2. Appears to act as a link between growth factor receptor signaling and the apoptotic pathways.
-
基因功能参考文献:
- This study shows for the first time that genetic knockout of Bad provides epileptic seizure protection in Kcna1-/- mice, a genetic model of epilepsy with sudden unexplained death. PMID: 29171006
- BAD knockout reduced epileptiform activity, and this effect was lost upon knockout or pharmacological inhibition of KATP channels. PMID: 29368690
- Bad is dispensable for TNF-mediated cell death. PMID: 25611386
- Results suggest that regulation of the proapoptotic activity of BAD plays a key role in the pathogenic mechanisms resulting in primary pigmented nodular adrenocortical disease tumor formation. PMID: 24865460
- fasting may increase the uptake beta-hydroxybutyrate by decreasing BAD in the brain during hypoglycemia PMID: 25043191
- Results indicate the downstream targets of insulin, cyclin D1, BAD, alpha-MHC, and GATA-4, elucidate a molecular mechanism of insulin in promoting cell proliferation and differentiation. PMID: 24020834
- our study suggests that Bad and Bmf co-regulate lymphocyte homeostasis and limit spontaneous transformation by mechanisms that may not exclusively be linked to the induction of lymphocyte apoptosis. PMID: 22430207
- Results reveal that IKK inhibits TNFalpha-induced apoptosis through two distinct but cooperative mechanisms: activation of the survival factor NF-kappaB and inactivation of the proapoptotic BH3-only BAD protein. PMID: 23332762
- RNAi-mediated silencing of STAT1 in soft tissue sarcoma (STS) cells was sufficient to increase expression of the apoptotic mediators Fas and Bad and to elevate the sensitivity of STS cells to Fas-mediated apoptosis PMID: 22805310
- BAD modulates counterregulatory responses to hypoglycemia and protective glucoprivic feeding PMID: 22162752
- the regulation of BAD by uremic toxins and report the sensitization of vascular smooth muscle cells to apoptosis upon BAD induction was explored. PMID: 22172950
- Tonicity-induced COX-2 expression and PGE2 synthesis in the renal medulla entails phosphorylation and inactivation of the pro-apoptotic protein Bad, thereby counteracting apoptosis in renal medullary epithelial cells. PMID: 21716255
- Caspase-3 is activated by the BAD-BAX cascade resulting in long term depression induction in the hippocampus. PMID: 21609830
- JNK1 is required for erythropoietin-mediated cell survival through phosphorylation and inactivation of the pro-apoptotic, Bcl-2 homology domain 3 (BH3)-only Bcl-associated death protein (Bad). PMID: 21095239
- Bad protein cooperate with bim protein in certain apoptotic responses and in the suppression of g-irradiation-induced thymic lymphoma.(Bad protein) PMID: 20431598
- Data show that loss of Bmf reduced the pressure to inactivate p53, whereas Bad deficiency did not, identifying Bmf as a novel component of the p53-independent tumor suppressor pathway triggered by c-Myc. PMID: 19965635
- The beta-arrestin 1-dependent ERK1/2 activation engaged by GLP-1 mediates the Ser-112 phosphorylation of Bad. PMID: 19915011
- The interaction of Bad with lipid rafts is a dynamic process regulated by IL-4 and involved in the control of apoptosis. PMID: 11907096
- activation by therapeutic inhibition of epidermal growth factor receptor and transactivation by insulin-like growth factor receptor PMID: 12011069
- Bcl-x(L) and Bcl-w target protein phosphatase 1alpha to Bad PMID: 12115603
- phosphorylation at serine 128 by activation of the JNK signaling pathway PMID: 12189144
- BAD phosphorylation protects cells from the deleterious effects of apoptotic stimuli and attenuates death pathway signaling by raising the threshold at which mitochondria release cytochrome c to induce cell death PMID: 12431371
- Bad apoptotic protein alone or in combination with bax apoptotic protein and the prostatic-specific promoter ARR(2)PB was an effective therapy for experimental prostatic neoplasms. PMID: 12490000
- Candida albicans phospholipomannan promotes survival of phagocytosed yeasts through modulation of of this protein's phosphorylation and macrophage apoptosis. PMID: 12551950
- HSV-1 US3 protein kinase blocks the caspases that cleave BAD at either residue 56 or 61 predicted to render the protein more proapoptotic or at residue 156, which would inactivate the protein. PMID: 12743316
- proapoptotic BAD suppresses tumorigenesis in the lymphocyte lineage PMID: 12876200
- combination of proteomics, genetics and physiology indicates an unanticipated role for BAD in integrating pathways of glucose metabolism and apoptosis PMID: 12931191
- PP2A dephosphorylation of pSer112 is the key initiating event regulating the activation of BAD during interleukin-3 withdrawal-induced apoptosis PMID: 12944463
- BAD is a substrate for pim-2 oncogene proto-oncogene PMID: 12954615
- regulation of Bad phosphorylation plays an active role in mediating anti-IgM-induced apoptosis of immature B cells PMID: 14585539
- JNK is required for IL-3-mediated cell survival through phosphorylation and inactivation of the proapoptotic Bcl-2 family protein BAD. PMID: 14967141
- Data show that the Bcl-2 homology 3 domain-only protein, Bad, is involved in cell death following IL-7 withdrawal from D1 cells, an IL-7-dependent murine thymocyte cell line. PMID: 15123689
- mechanisms that regulate the conversion of BAD from an anti-death to a pro-death factor include alternative splicing that produces N-terminally truncated BAD(S)and conversion by caspases into a pro-death fragment that resembles the short splice variant PMID: 15231831
- alteration of lipid rafts is an early event in the apoptotic cascade indirectly induced by interleukin-4 deprivation via PP1alpha activation, dephosphorylation of cytoplasmic Bad, and caspase activation PMID: 15634756
- Bad phosphorylation is not essential for PKB-mediated survival signaling in hemopoietic cells PMID: 15843895
- Pak1-dependent Raf-1 phosphorylation regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association PMID: 15849194
- BAD induces apoptosis upon detecting the coincidence of G2/M phase and growth factor deprivation PMID: 15901741
- phosphorylation of BAD Serine 128 exerts cell-specific effects on apoptosis PMID: 15907327
- All three Pim kinase family members predominantly phosphorylate Bad on Ser112 and in addition are capable of phosphorylating Bad on multiple sites associated with the inhibition of the pro-apoptotic function of Bad in HEK-293 cells PMID: 16403219
- cellular cholesterol biosynthesis is critical for the activation and maintenance of the Akt-Bad cell survival cascade in response to growth factors such as insulin. PMID: 16513830
- These data establish a connection between calcium overload and mitochondria-mediated death pathways in outer hair cells and also suggest a dual role for BAD. PMID: 16521126
- the interaction of BAD with membranes is tied to binding of 14-3-3 protein and activation and membrane translocation of Bcl-XL PMID: 16603546
- Study shows, using spectroscopic methods, that the BH3-only proteins Bim, Bad and Bmf are unstructured in the absence of binding partners. PMID: 16645638
- Bad was not required for cell death following IL-3 withdrawal, suggesting changes to phosphorylation of Bad play only a minor role in apoptosis. PMID: 16705087
- Both gonadotropin releasing hormone andepidermal growth factor (EGF) caused rapid phosphorylation of BAD. PMID: 16741954
- The proapoptotic protein Bad is a key player in cell survival decisions, and is regulated post-translationally by several signaling networks. PMID: 17535812
- Raf-1 in beta-cells led to a striking loss of Bad phosphorylation at serine 112 and an increase in the protein levels of both Bad and Bax PMID: 18006502
- These findings provide genetic proof of the bifunctional activities of BAD in both beta cell survival and insulin secretion. PMID: 18223655
- Thr-201 phosphorylation of Bad by JNK1 is required for PFK-1 activation PMID: 18469002
- BAD is the only BCL-2 family protein expressed in parietal cells PMID: 18779780
显示更多
收起更多
-
亚细胞定位:Mitochondrion outer membrane. Cytoplasm.
-
蛋白家族:Bcl-2 family
-
数据库链接:
KEGG: mmu:12015
STRING: 10090.ENSMUSP00000025910
UniGene: Mm.4387
Most popular with customers
-
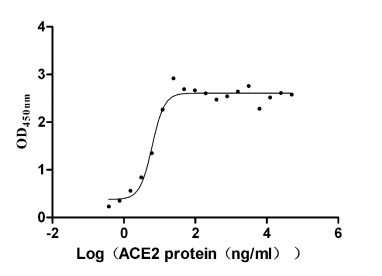
Recombinant Paguma larvata Angiotensin-converting enzyme 2 (ACE2), partial (Active)
Express system: Mammalian cell
Species: Paguma larvata (Masked palm civet)
-
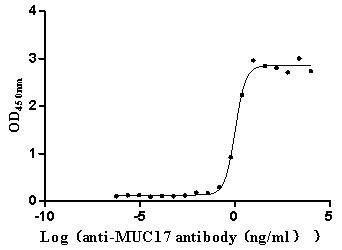
Recombinant Human Mucin-17 (MUC17), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
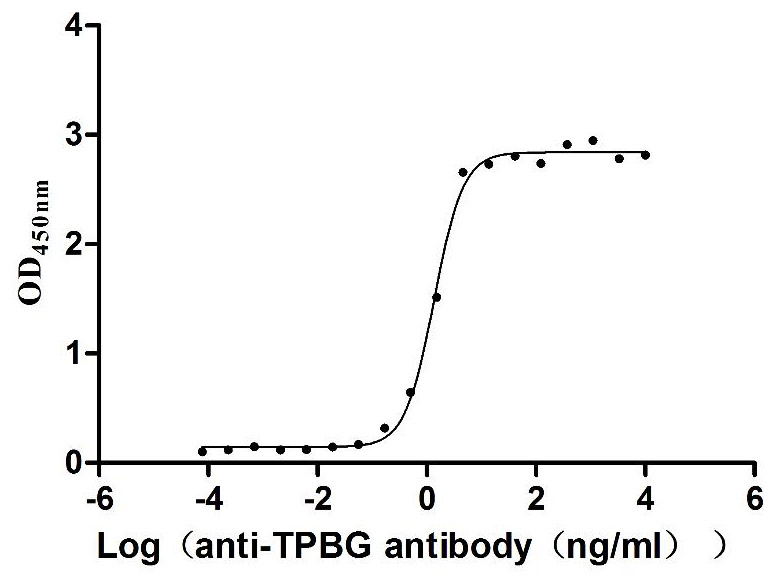
Recombinant Human Trophoblast glycoprotein (TPBG), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
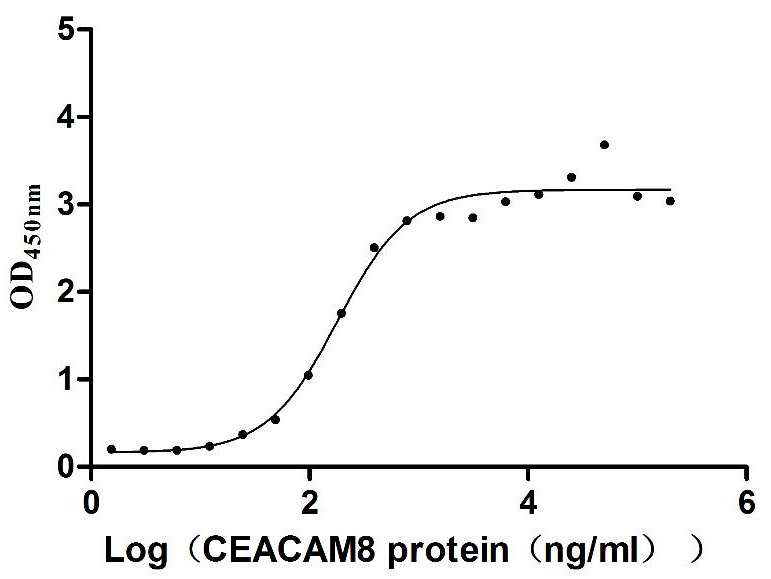
Recombinant Human Carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human C-C chemokine receptor type 8 (CCR8)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
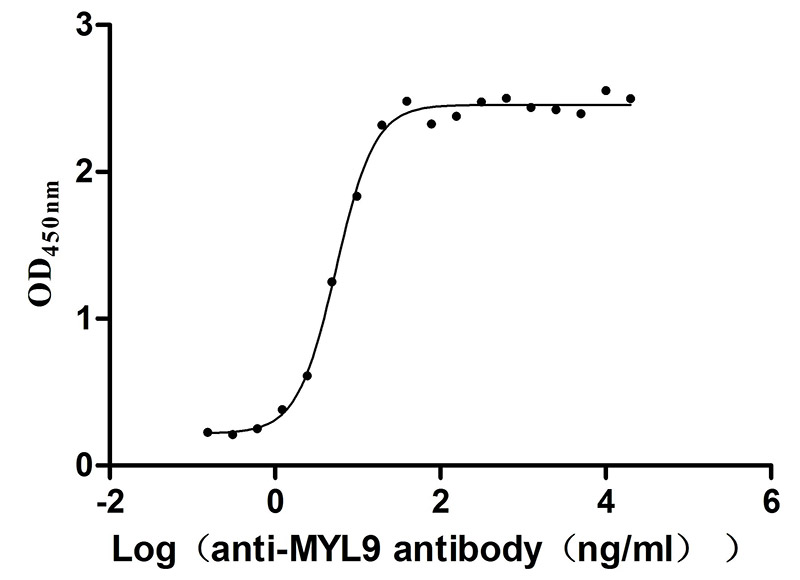
Recombinant Human Myosin regulatory light polypeptide 9 (MYL9) (Active)
Express system: Yeast
Species: Homo sapiens (Human)
-
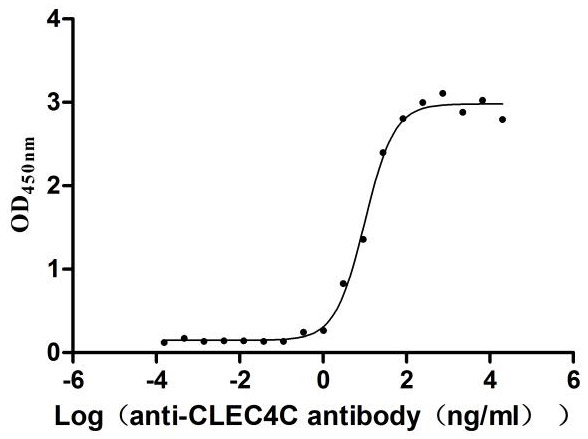
Recombinant Human C-type lectin domain family 4 member C (CLEC4C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)