Recombinant Mouse Heat shock 70 kDa protein 1B (Hspa1b)
-
货号:CSB-YP010822MO
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP010822MO-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP010822MO
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP010822MO
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:Hspa1b
-
Uniprot No.:
-
别名:Hspa1b; Hcp70.1; Hsp70-1; Hsp70a1; Hspa1; Heat shock 70 kDa protein 1B; Heat shock 70 kDa protein 1; HSP70.1
-
种属:Mus musculus (Mouse)
-
蛋白长度:Full Length of Mature Protein
-
表达区域:2-642
-
氨基酸序列AKNTAIGID LGTTYSCVGV FQHGKVEIIA NDQGNRTTPS YVAFTDTERL IGDAAKNQVA LNPQNTVFDA KRLIGRKFGD AVVQSDMKHW PFQVVNDGDK PKVQVNYKGE SRSFFPEEIS SMVLTKMKEI AEAYLGHPVT NAVITVPAYF NDSQRQATKD AGVIAGLNVL RIINEPTAAA IAYGLDRTGK GERNVLIFDL GGGTFDVSIL TIDDGIFEVK ATAGDTHLGG EDFDNRLVSH FVEEFKRKHK KDISQNKRAV RRLRTACERA KRTLSSSTQA SLEIDSLFEG IDFYTSITRA RFEELCSDLF RGTLEPVEKA LRDAKMDKAQ IHDLVLVGGS TRIPKVQKLL QDFFNGRDLN KSINPDEAVA YGAAVQAAIL MGDKSENVQD LLLLDVAPLS LGLETAGGVM TALIKRNSTI PTKQTQTFTT YSDNQPGVLI QVYEGERAMT RDNNLLGRFE LSGIPPAPRG VPQIEVTFDI DANGILNVTA TDKSTGKANK ITITNDKGRL SKEEIERMVQ EAERYKAEDE VQRDRVAAKN ALESYAFNMK SAVEDEGLKG KLSEADKKKV LDKCQEVISW LDSNTLADKE EFVHKREELE RVCSPIISGL YQGAGAPGAG GFGAQAPPKG ASGSGPTIEE VD
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation. This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones. The co-chaperones have been shown to not only regulate different steps of the ATPase cycle, but they also have an individual specificity such that one co-chaperone may promote folding of a substrate while another may promote degradation. The affinity for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. It goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. The co-chaperones are of three types: J-domain co-chaperones such as HSP40s (stimulate ATPase hydrolysis by HSP70), the nucleotide exchange factors (NEF) such as BAG1/2/3 (facilitate conversion of HSP70 from the ADP-bound to the ATP-bound state thereby promoting substrate release), and the TPR domain chaperones such as HOPX and STUB1. Maintains protein homeostasis during cellular stress through two opposing mechanisms: protein refolding and degradation. Its acetylation/deacetylation state determines whether it functions in protein refolding or protein degradation by controlling the competitive binding of co-chaperones HOPX and STUB1. During the early stress response, the acetylated form binds to HOPX which assists in chaperone-mediated protein refolding, thereafter, it is deacetylated and binds to ubiquitin ligase STUB1 that promotes ubiquitin-mediated protein degradation. Regulates centrosome integrity during mitosis, and is required for the maintenance of a functional mitotic centrosome that supports the assembly of a bipolar mitotic spindle. Enhances STUB1-mediated SMAD3 ubiquitination and degradation and facilitates STUB1-mediated inhibition of TGF-beta signaling. Essential for STUB1-mediated ubiquitination and degradation of FOXP3 in regulatory T-cells (Treg) during inflammation.
-
基因功能参考文献:
- binding of IL-5 to IL-5Ralpha receptors enhances angiogenic responses by stimulating the expression of HSP70-1 via the eNOS signaling pathway. PMID: 28317868
- The present study clearly demonstrated that the ectromelia virus infection upregulates the expression of Hspa1b in order to promote its replication. PMID: 29749430
- HSP70 expression is up-regulated in fatty liver. PMID: 29631603
- CM-695, a small molecule that induces the expression of the HSPA1A/B genes, increases the vessel wall levels of Hsp70 and prevents thrombosis at least as efficiently as rivaroxaban without increasing bleeding risk. PMID: 28837204
- mHSP70407-426 could assist VEGF to display enhanced anti-tumor effects, which are important for the further application of mHSP70407-426 to enhance antigen-specific immune responses PMID: 29572180
- Extracellular Hsp70 and Hsp90, either in soluble form or secreted as part of exosomes from tumor cells, are responsible for tumor induction of cachexia. PMID: 28928431
- HSP70 functions as a negative regulator in the TGF-beta- stimulated VEGF synthesis in osteoblasts, and that the suppressive effect of HSP70 is exerted via regulation of p38 MAP kinase. PMID: 29179216
- The data implicate an involvement of Hsp70 oxidatively damaged protein degradation by the 20S proteasome. PMID: 27498116
- interaction between BAG3 and HSP70 is essential for BAG3 to stabilize small heat shock proteins and maintain cardiomyocyte protein homeostasis PMID: 28737513
- Data show that Klotho protein is present in the heart, and reduced levels of myocardial Klotho in aging mice is accompanied by lower levels heat shock protein 70 (HSP70) in the myocardium. PMID: 28152512
- analysis of the intracellular pathways implicated in Hsp70 regulated signal transduction showed the involvement of both PI3K/AKT and NF-kappaB. PMID: 27925208
- HSPA1A/B induction is a novel approach to delay thrombus formation with minimal bleeding risk in knockout mice PMID: 26976620
- Hsp70 expression was observed trophoblast cells and decidual cells and was relatively constant throughout the pregnancy. PMID: 26799792
- The results of comparative analysis of interaction between the protein cytotoxic complex Tag 7-Hsp70 and the Tag 7 component of this complex with TNFR1 receptor in solution and in tumor cells are presented. PMID: 27021371
- the protective effect of HSP70 may be associated with inhibition of NF-kappaB and stimulation of NOS/NO signaling pathways PMID: 26215736
- activation of the HSP70-TLR4 axis, stimulated at least in part by albumin, in the tubular cell is a newly identified mechanism associated with induction of tubulointerstitial inflammation. PMID: 26398934
- Knockdown lines were created for specific DSBs in regions of the chromosome that are coding for HSPA1B. Clonogenic cell survival was significantly lower in irradiated Hsp70 KD cells with low mHsp70 expression, than in ctrl cells. PMID: 26197988
- Hsp70.1-deficient mice were more resistant to developing experimental autoimmune encephalomyelitis (EAE) compared with their wild-type littermates, suggesting Hsp70.1 plays a role in promoting myelin oligodendrocyte glycoprotein specific T cell response. PMID: 25153885
- We report exosomal Hsp70 can expand and induce the activation of myeloid-derived suppressor cells PMID: 25603952
- Results suggest that overexpression of Hsp70 may protect against brain ischemia via an anti-inflammatory mechanism by interrupting the phosphorylation of upstream of transcription factors. PMID: 25485480
- These findings indicate that in mice HSP27 and HSP70 play a key role in the induction of cell-mediated immunity to carcinogenic polyaromatic hydrocarbons PMID: 25840912
- Tumor-derived inducible heat-shock protein 70 (HSP70) is an essential component of anti-tumor immunity. PMID: 24662819
- Hsp70 interacts with acidic glycopolymers that contain clustered sulfated and di-sialylated glycan moieties on a polyacrylamide backbone, but not with neutral or mono-sialylated glycopolymers. PMID: 24909693
- HSP70 has a role in liver regeneration after partial hepatectomy in mice PMID: 24357103
- It plays a protective role against AP-induced liver injury. PMID: 24219791
- BAG-1 possesses an ubiquitin-like domain (Ub-LD) responsible for proteasomal association and for promoting substrate release from Hsc70/Hsp70 in vitro by accelerating the chaperone ATP/ADP exchange rate. PMID: 23178238
- Functional study of Hsp70 revealed that decreased expression of Hsp70 diminished the apoptosis induced by Sialic acid-binding lectin. It is suggested that Hsp70 participates in the antitumor effect of Sialic acid-binding lectin. PMID: 24173532
- Exercise preconditioning in mice can prevent oxidative stress and apoptotic cell death in the kidney resulting from ischemic injury even under Hsp70 deficiency PMID: 23780557
- Loss of the inducible Hsp70 delays the inflammatory response to skeletal muscle injury and severely impairs muscle regeneration. PMID: 23626847
- study reports that carbonylation of HSP70 by reactive oxygen species is associated with the pathogenesis of contact hypersensitivity, suggesting possibility of HSP70-targeting therapy in contact hypersensitivity PMID: 23679814
- Hsp70 is crucially involved in the labile phase of development of behavioral sensitization induced by a single morphine exposure, probably functioning as a molecular chaperone. PMID: 22647551
- Inner ear supporting cell secreted HSP70 protects hair cells from apoptotic cell death. PMID: 23863716
- Hsp70 is required for IKK activation and STAT1/IRF-1 promoter binding amid iNOS gene transactivation. PMID: 23419754
- We confirmed that Hsp70 and TLR4 coimmunoprecipitate in lung tissue and MLECs. Hsp70-mediated NF-kappaB activation appears to depend upon TLR4. In the absence of TLR4, Hsp70 loses its protective effects in endothelial cells. PMID: 23817427
- Acetylation of hsp70 Regulates SUMOylation of Vps34 and Its Association with Beclin 1 in Breast Cancer Cells PMID: 23569248
- Formation of HSP70- and MDM2-dependent protein coaggregates in tumours with high levels of these two proteins could be one of the mechanisms by which mutant p53 is stabilized. PMID: 23251530
- Hsp70 induction is sufficient to prevent NRG1-induced demyelination by enhancing the proteasomal degradation of c-Jun. PMID: 23240583
- This study provides evidence for an inhibitory effect of HSP70 on UV-induced wrinkle formation. PMID: 23096703
- Collectively, these results support a novel axis of type I interferon-dependent antiviral immunity in the measles virus-infected brain that is driven by hsp70. PMID: 23135720
- Opposing actions of heat shock protein 90 and 70 regulate nicotinamide adenine dinucleotide phosphate oxidase stability and reactive oxygen species production. PMID: 23023377
- Higher sensitivity of ES cells to proteotoxic stress may be related with lower capacity of HSP70 expression. PMID: 22617454
- cholesterol and more likely 7beta-OH may exert their pro-atherogenic effects by lowering hsp70 protein production and inhibiting glutathione synthesis by macrophages present in the arterial wall PMID: 22896903
- effect of oxidative stress on the process of spermatogenesis in terms of hsp70 expression was studied. For creating different oxidative PMID: 22900316
- Heat shock protein 70 (rSj648/hsp70) induces high protection against schistosome-infection contributing to predominant Th1 reaction and the correlation with high expression of IFN-gamma. PMID: 22590864
- The data herein supports the theory that HSP70 is involved in normal skin protein configuration and the cellularity of early wound healing. PMID: 22275297
- The Hsp70 inhibits H(2)O(2)-induced nucleolar fragmentation through the translocation of Hsp70 into nucleolar and its protection against impairment of nucleolin. PMID: 21960124
- There was a significant induction of HSP70 in the arthritic chondrocytes treated with laser therapy. PMID: 21749827
- Extracellular HSP70 acting via TLR2 and its obligate downstream adaptor molecule, MyD88, activate NFkappaB, causing cardiomyocyte inflammation and decreased contractility. PMID: 21817814
- HSP70i was necessary and sufficient to accelerate depigmentation in vitiligo-prone Pmel-1 mice, accompanied by lasting phenotypic changes in dendritic cell subpopulations. PMID: 21978301
- These results indicate that HSP70 alone is not sufficient to reduce MPTP-induced dopaminergic neuronal damage. PMID: 21782904
显示更多
收起更多
-
亚细胞定位:Cytoplasm. Cytoplasm, cytoskeleton, microtubule organizing center, centrosome.
-
蛋白家族:Heat shock protein 70 family
-
组织特异性:Testis-specific.
-
数据库链接:
KEGG: mmu:15511
STRING: 10090.ENSMUSP00000128525
UniGene: Mm.372314
Most popular with customers
-
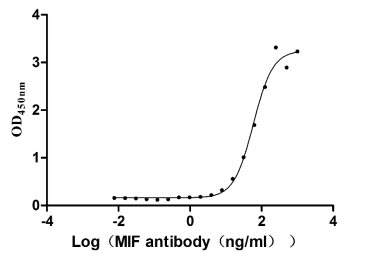
Recombinant Human Macrophage migration inhibitory factor (MIF) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
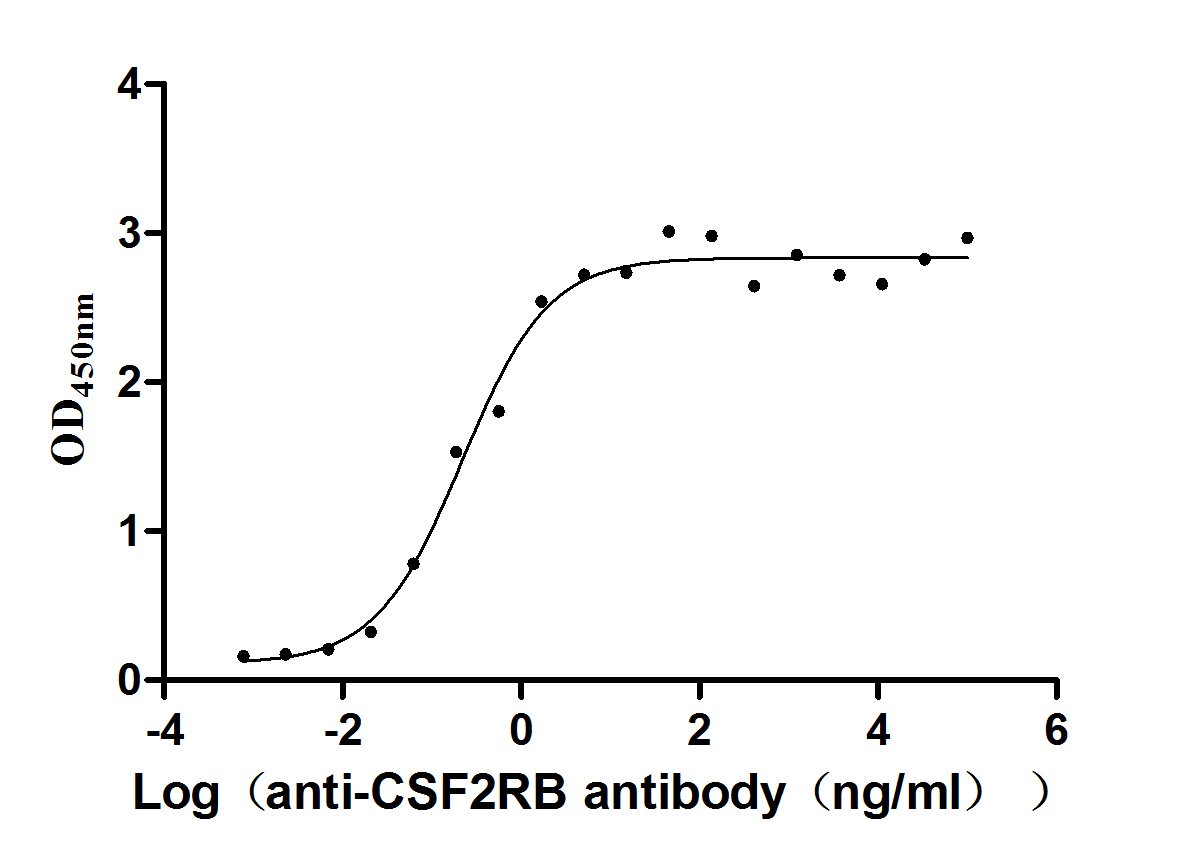
Recombinant Human Cytokine receptor common subunit beta (CSF2RB), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
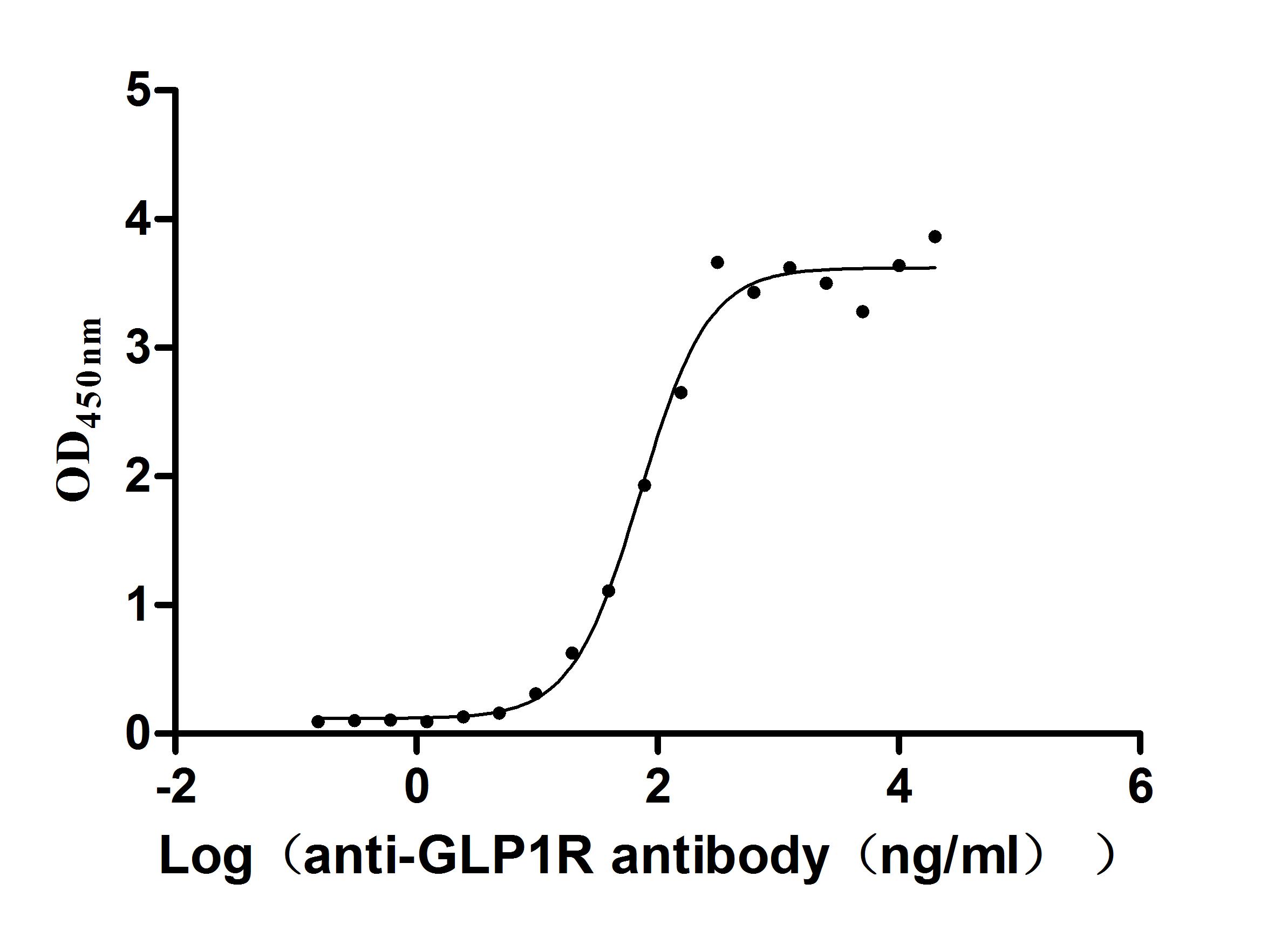
Recombinant Human Glucagon-like peptide 1 receptor (GLP1R), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
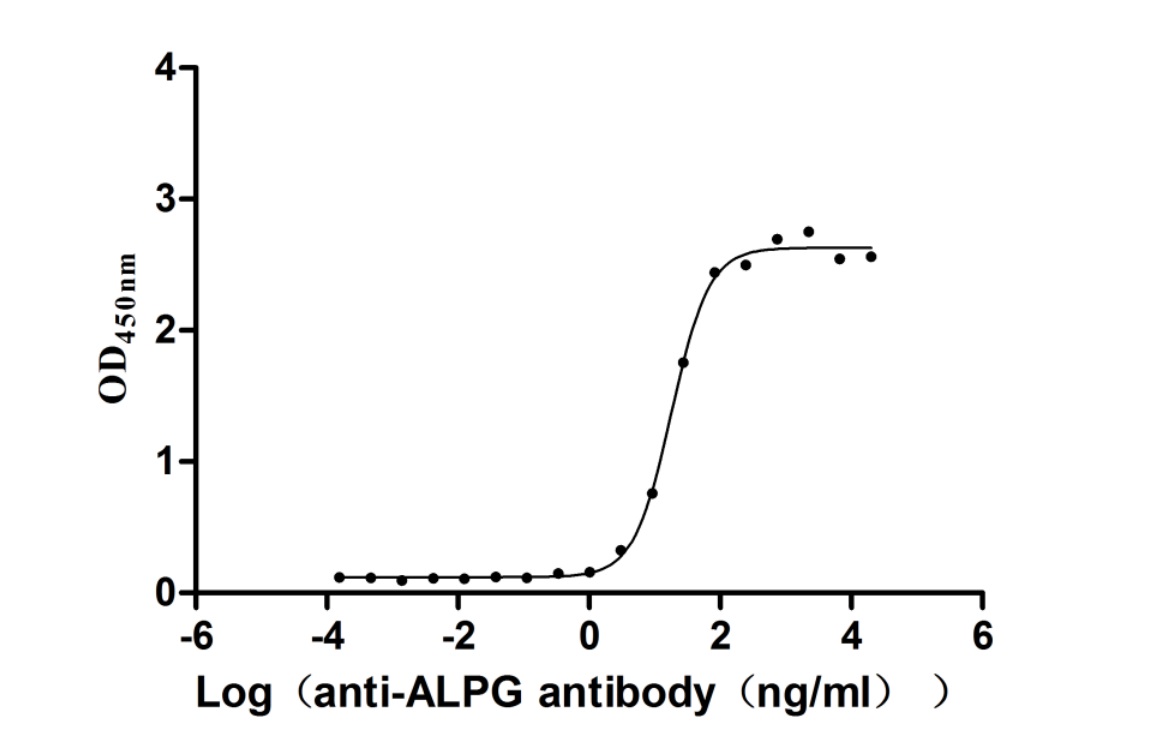
Recombinant Human Alkaline phosphatase, germ cell type (ALPG) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
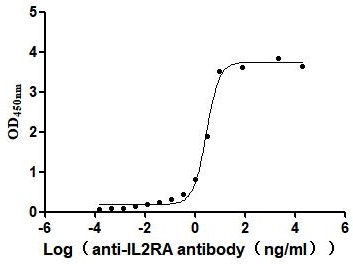
Recombinant Human Interleukin-2 receptor subunit alpha (IL2RA), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
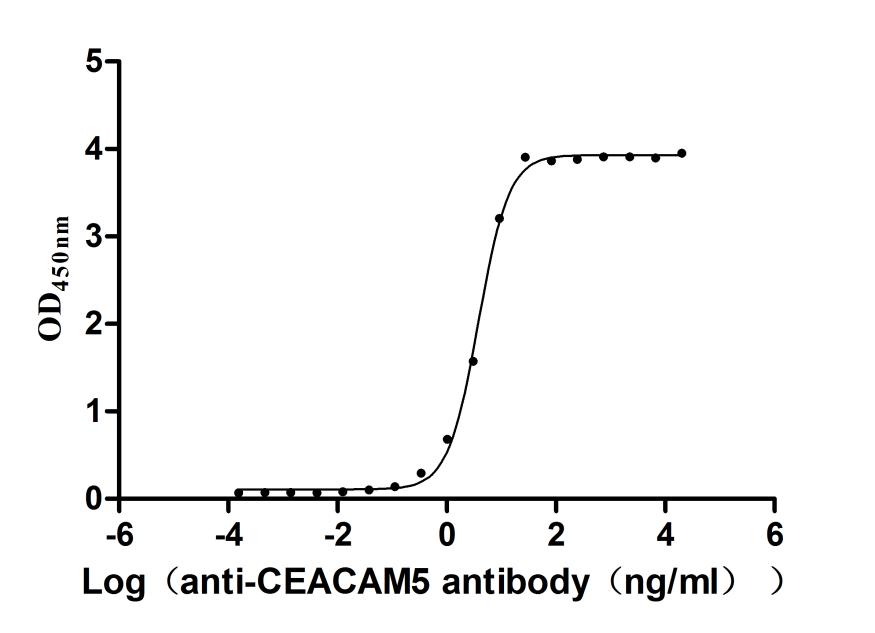
Recombinant Human Carcinoembryonic antigen-related cell adhesion molecule 8(CEACAM8) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
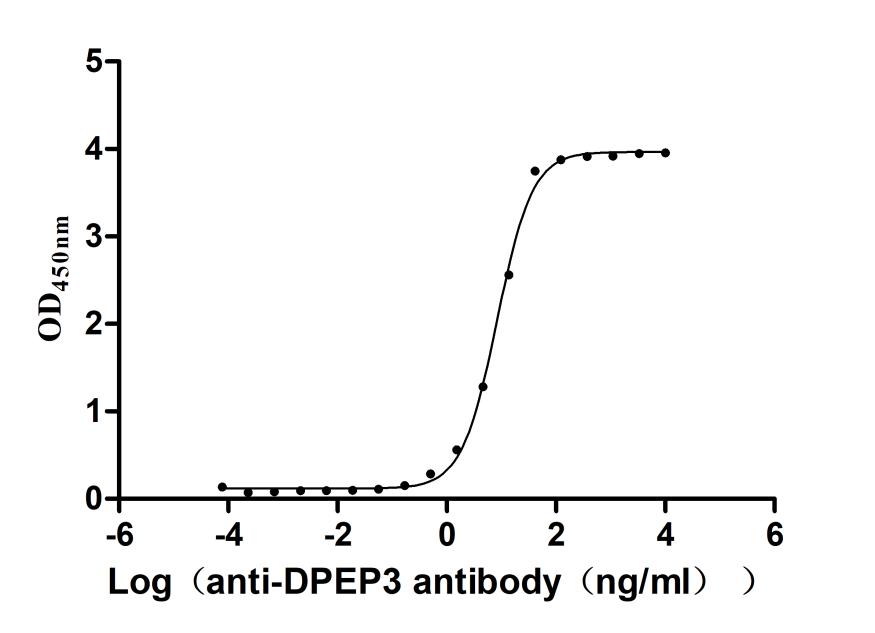
Recombinant Macaca fascicularis Dipeptidase 3(DPEP3) (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Express system: Mammalian cell
Species: Macaca mulatta (Rhesus macaque)