Recombinant Rat Heat shock protein HSP 90-alpha (Hsp90aa1)
-
货号:CSB-YP010802RA
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP010802RA
-
规格:
-
来源:E.coli
-
其他:
-
货号:CSB-EP010802RA-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP010802RA
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP010802RA
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
别名:Hsp90aa1; Hsp86; HspcaHeat shock protein HSP 90-alpha; Heat shock 86 kDa; HSP 86; HSP86
-
种属:Rattus norvegicus (Rat)
-
蛋白长度:Full Length of Mature Protein
-
表达区域:2-733
-
氨基酸序列PEETQTQDQ PMEEEEVETF AFQAEIAQLM SLIINTFYSN KEIFLRELIS NSSDALDKIR YESLTDPSKL DSGKELHINL IPNKQDRTLT IVDTGIGMTK ADLINNLGTI AKSGTKAFME ALQAGADISM IGQFGVGFYS AYLVAEKVTV ITKHNDDEQY AWESSAGGSF TVRTDTGEPM GRGTKVILHL KEDQTEYLEE RRIKEIVKKH SQFIGYPITL FVEKERDKEV SDDEAEEKEE KEEEKEKEEK ESDDKPEIED VGSDEEEEEK KDGDKKKKKK IKEKYIDQEE LNKTKPIWTR NPDDITNEEY GEFYKSLTND WEEHLAVKHF SVEGQLEFRA LLFVPRRAPF DLFENRKKKN NIKLYVRRVF IMDNCEELIP EYLNFIRGVV DSEDLPLNIS REMLQQSKIL KVIRKNLVKK CLELFTELAE DKENYKKFYE QFSKNIKLGI HEDSQNRKKL SELLRYYTSA SGDEMVSLKD YCTRMKENQK HIYFITGETK DQVANSAFVE RLRKHGLEVI YMIEPIDEYC VQQLKEFEGK TLVSVTKEGL ELPEDEEEKK KQEEKKTKFE NLCKIMKDIL EKKVEKVVVS NRLVTSPCCI VTSTYGWTAN MERIMKAQAL RDNSTMGYMA AKKHLEINPD HSIIETLRQK AEADKNDKSV KDLVILLYET ALLSSGFSLE DPQTHANRIY RMIKLGLGID EDDPTVDDTS AAVTEEMPPL EGDDDTSRME EVD
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity which is essential for its chaperone activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function. Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself. Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co-chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle. Plays a critical role in mitochondrial import, delivers preproteins to the mitochondrial import receptor TOMM70. Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels. In the first place, they alter the steady-state levels of certain transcription factors in response to various physiological cues. Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment. Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression. Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes. Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation. Mediates the association of TOMM70 with IRF3 or TBK1 in mitochodria outer membrane which promotes host antiviral response.
-
亚细胞定位:Nucleus. Cytoplasm. Melanosome. Cell membrane. Mitochondrion.
-
蛋白家族:Heat shock protein 90 family
-
数据库链接:
KEGG: rno:103692716
STRING: 10116.ENSRNOP00000009556
UniGene: Rn.119867
Most popular with customers
-
Recombinant Human T-cell antigen CD7 (CD7), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
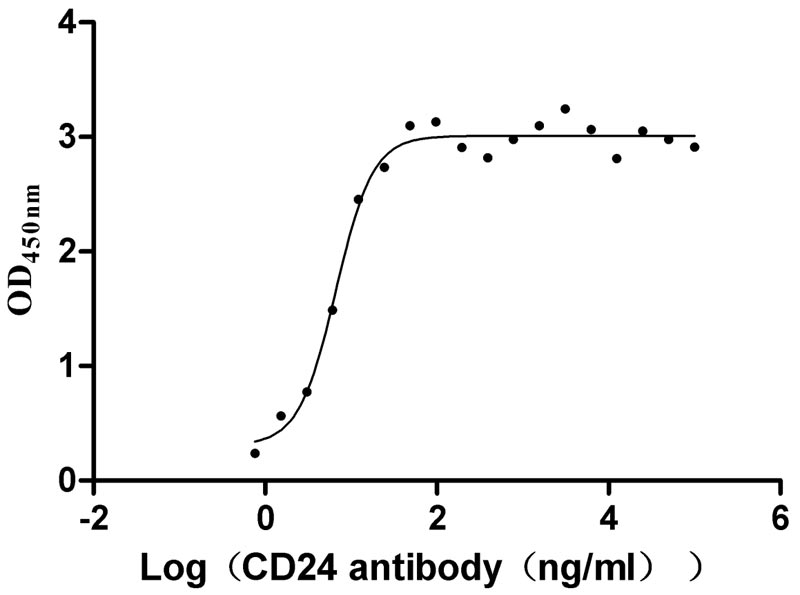
Recombinant Human Signal transducer CD24 (CD24)-Nanoparticle (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Mouse Claudin-18 (Cldn18)-VLPs (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
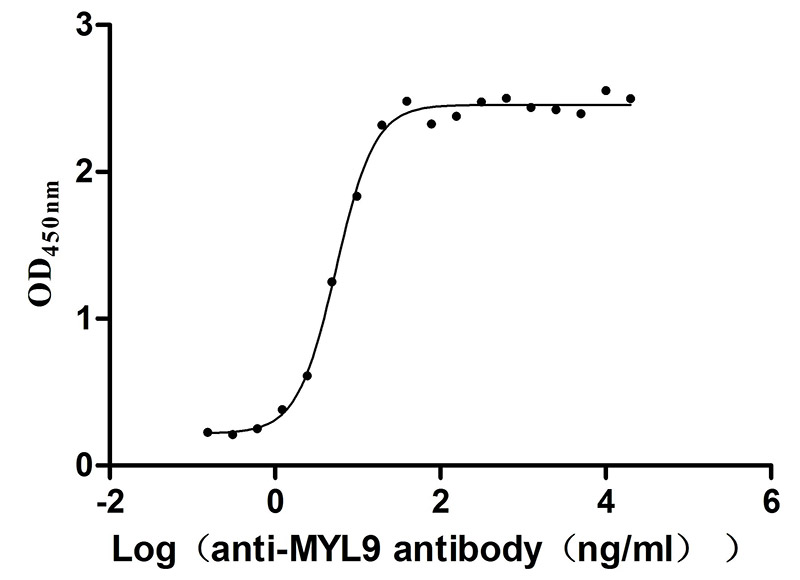
Recombinant Human Myosin regulatory light polypeptide 9 (MYL9) (Active)
Express system: Yeast
Species: Homo sapiens (Human)


-AC1.jpg)