Recombinant Rat Major prion protein (Prnp)
-
中文名称:大鼠Prnp重组蛋白
-
货号:CSB-YP018739RA
-
规格:¥1836
-
图片:
-
其他:
产品详情
-
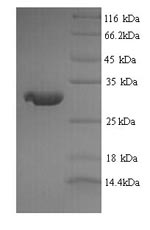
纯度:Greater than 90% as determined by SDS-PAGE.
-
基因名:Prnp
-
Uniprot No.:
-
别名:Prnp; Prn; Prp; Major prion protein; PrP; CD antigen CD230
-
种属:Rattus norvegicus (Rat)
-
蛋白长度:Full Length of Mature Protein
-
来源:Yeast
-
分子量:24.3kDa
-
表达区域:29-231aa
-
氨基酸序列GGWNTGGSRYPGQGSPGGNRYPPQSGGTWGQPHGGGWGQPHGGGWGQPHGGGWGQPHGGGWSQGGGTHNQWNKPSKPKTNLKHVAGAAAAGAVVGGLGGYMLGSAMSRPMLHFGNDWEDRYYRENMYRYPNQVYYRPVDQYSNQNNFVHDCVNITIKQHTVTTTTKGENFTETDVKMMERVVEQMCVTQYQKESQAYYDGRRS
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal 6xHis-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:Tris-based buffer,50% glycerol
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:Its primary physiological function is unclear. May play a role in neuronal development and synaptic plasticity. May be required for neuronal myelin sheath maintenance. May promote myelin homeostasis through acting as an agonist for ADGRG6 receptor. May play a role in iron uptake and iron homeostasis. Soluble oligomers are toxic to cultured neuroblastoma cells and induce apoptosis (in vitro). Association with GPC1 (via its heparan sulfate chains) targets PRNP to lipid rafts. Also provides Cu(2+) or ZN(2+) for the ascorbate-mediated GPC1 deaminase degradation of its heparan sulfate side chains.
-
基因功能参考文献:
- the mechanism by which stressful stimuli induced by opiate withdrawal and the subsequent long-term homeostatic changes in hippocampal plasticity, modulate the expression and the dynamics of Prion protein. PMID: 28081197
- The results suggest a role of PrP(C) in proteostasis, dysfunctions of which may be involved in the pathogenesis of neurodegenerative diseases such as TSE and Alzheimer's Disease. PMID: 26946358
- this study implies that lithium, the commonly used mood stabilizer, may be a promising therapeutic agent in TSE, particularly in case of the disease forms associated with accumulation of cytoPrP. PMID: 26149502
- We provide the first report that mean PrPC concentration in primary blast exposed rats is significantly increased compared with controls PMID: 25058115
- data provide new insights into the cellular mechanism of prion conversion and suggest that GM1-prion protein interaction at the cell surface could play a significant role in the mechanism predisposing to pathology. PMID: 24859148
- The toxicity of PrP(111-126) to cultured astrocytes was reduced following the addition of Cu(2+) ions, owing to binding affinity of copper towards histidine moiety present in the peptide. PMID: 24386462
- Down regulation of brain cellular prion protein in an animal model of insulin resistance. PMID: 24780399
- PrP(106-126) cytotoxic amyloid oligomers disruption of membranes is dependent on bilayer composition PMID: 24554723
- Prion protein-mediated toxicity of amyloid-beta oligomers requires lipid rafts and the transmembrane LRP1 PMID: 23386614
- The study suggests a pivotal role for PrP(C) in the cell adaptation to copper limitation through a direct activity of ion uptake. PMID: 22362149
- The prion protein and mrna was regulaion in vitamin B(12)rats. PMID: 22116041
- The nerves of the PrP(C)-treated rats show typical cobalamin-deficient lesions, significantly decreased abnormal maximum nerve conduction velocity values, and significantly increased tumor necrosis factor-alpha levels. PMID: 22102467
- mutant or cytosolic PrP expression in transgenic mice and human or rat cells is not associated with endoplasmic reticulum stress or proteasome dysfunction PMID: 21559407
- Data demonstrate that PrP(C) is abundant in the nuclear lamina of endocrine and neuronal cells and interacts with histone H1(0), histone H3 and lamin B1. PMID: 21277044
- These new data indicate that PrPc gene regulation is highly dependent on disruption of chromatin fiber assembly, which allows some ubiquitous transcription factors accession to specific DNA elements PMID: 11739375
- In retinal explants from neonatal mice and rats, engagement of PrP(c) transduces neuroprotective signals through a cAMP/PKA-dependent pathway. PrP(c) may function as a trophic receptor, the activation of which leads to a neuroprotective state. PMID: 12093733
- Two heat-shock elements (HSEs) were located at the positions of -680 bp (HSE1; GGAACTATTCTTGACATTGCT), and -1653 bp (HSE2; TGAGAACTCAGGAAG) of the rat PrP (RaPrP) gene promoter. PMID: 12392052
- PrP is not subject to retrotranslocation from the ER into the cytoplasm prior to degradation by the proteasome PMID: 12663673
- Various prion glycoforms in B104 neuroblastoma cells were tightly regulated as a function of cell density and during neuronal differentiation. Potential role of cellular prion protein in cell-cell interactions and differentiation. PMID: 12911750
- the intrinsic properties of PrP-(82-146) are dependent upon the integrity of the C-terminal region and account for the massive deposition of PrP amyloid in GSS PMID: 12970341
- Glycosylphosphatidylinositol-anchored prion protein and Thy-1 were largely clustered in separate domains on the neuronal surface; the lipid content of these membrane domains differed markedly PMID: 14660659
- Prion proteins are membrane protein, isolated from neuronal cells, along with Marcks and fyn. PMID: 14741357
- PRNP shows no direct CAGA box correspondence with the APP superfamily members. PMID: 15208260
- early association of PrP(C) with cholesterol-enriched rafts facilitates its correct folding PMID: 15229281
- We propose that this unusual beta-turn-rich form of PrP may be a precursor of PrPSc and a candidate for the neurotoxic molecule in prion pathogenesis. PMID: 15670783
- Immunostained cells expressing PrP(c) appear scattered throughout the epithelium of fundic and pyloric glands as well as in intestinal villi and crypts. PMID: 15891070
- These results indicate that DRMs are essential for proper trafficking and distribution of PrP(C) at late stages of neuronal differentiation and that its function, at least in hippocampus, is restricted to the axonal domain. PMID: 16139509
- Prnp expression is upregulated by copper in neuronal cells by an MTF-1-independent mechanism, and suggest a metal-specific modulation of Prnp in neurons PMID: 16148034
- prion protein plasma membrane environment in differentiated neurons resulted to be a complex entity, whose integrity requires a network of lipid-mediated non-covalent interactions PMID: 16248888
- Our results indicated a novel PrP(C) interacting protein and suggested that this complex might be relevant in modulating a variety of electrophysiological-dependent cellular responses PMID: 16750514
- J protein family serves as a 'folding catalyst' for PrP(C) and implicates Rdj2 as a factor in the protection against prion diseases PMID: 16774738
- Pronounced cytosolic aggregation of cellular prion protein in pancreatic beta-cells in response to hyperglycemia. PMID: 17146448
- Our findings show that, through its interaction with LN, hippocampal PrPc plays a critical role in memory processing and suggest that this role is mediated by activation of both PKA and ERK1/2 signaling pathways. PMID: 17156386
- PrP(106-126) aggregates in vitro and this aggregation state is important for its neurotoxicity PMID: 17405933
- the plasma membrane localization of PrP(C) and/or of the converted scrapie form might be necessary for the development of a symptomatic disease PMID: 17556367
- PrP(C) is widely expressed in a subset of neurons that contain markers of inhibitory populations of cells throughout the rat brain PMID: 17854776
- Results demonstrate by immunohistochemical analysis the colocalization of neuroglobin and PrP(c) in the retinal ganglion cell layer. PMID: 19327369
- Our data support a significant role for PrP(c) as a response mediator in neuritogenesis and cell differentiation. PMID: 19457127
- The peculiar lipid composition and, in particular, the presence of proteins involved in synaptic plasticity, cell adhesion, cytoskeleton regulation and signaling suggest an important physiological role for the PrPC prion domain in neurons. PMID: 19493159
- Lipid rafts and clathrin cooperate in the internalization of PrP in epithelial FRT cells PMID: 19503793
- distinct N-terminal cleavage products of PrP(c) harbor different biological activities underlying the various phenotypes linking PrP(c) to cell survival. PMID: 19850936
显示更多
收起更多
-
相关疾病:Found in high quantity in the brain of humans and animals infected with degenerative neurological diseases such as kuru, Creutzfeldt-Jakob disease (CJD), Gerstmann-Straussler syndrome (GSS), scrapie, bovine spongiform encephalopathy (BSE), transmissible mink encephalopathy (TME), etc.
-
亚细胞定位:Cell membrane; Lipid-anchor, GPI-anchor. Golgi apparatus.
-
蛋白家族:Prion family
-
数据库链接:
KEGG: rno:24686
STRING: 10116.ENSRNOP00000028881
UniGene: Rn.3936
Most popular with customers
-
Recombinant Human Signal transducer CD24 (CD24)-Nanoparticle (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Microtubule-associated protein tau (MAPT) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
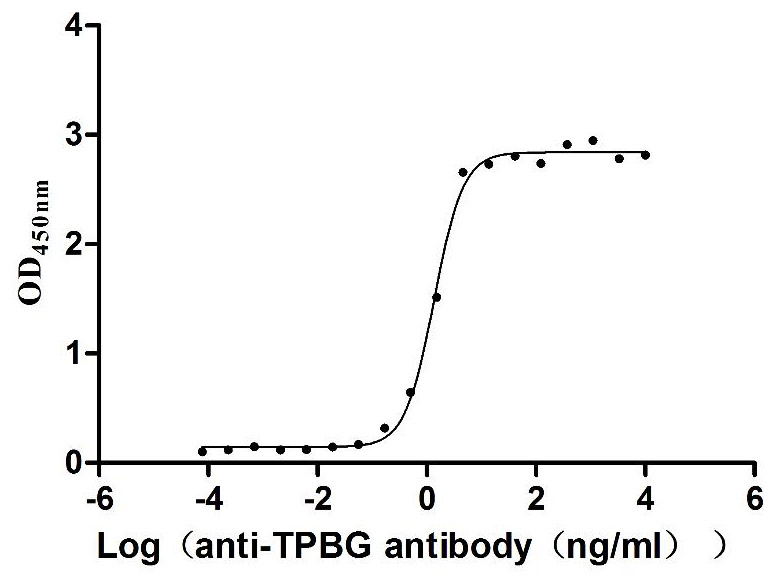
Recombinant Human Trophoblast glycoprotein (TPBG), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
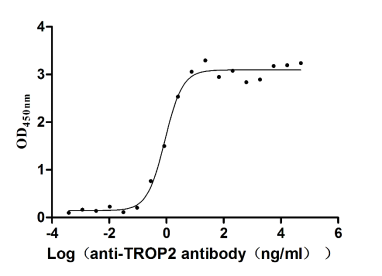
Recombinant Human Tumor-associated calcium signal transducer 2 (TACSTD2), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
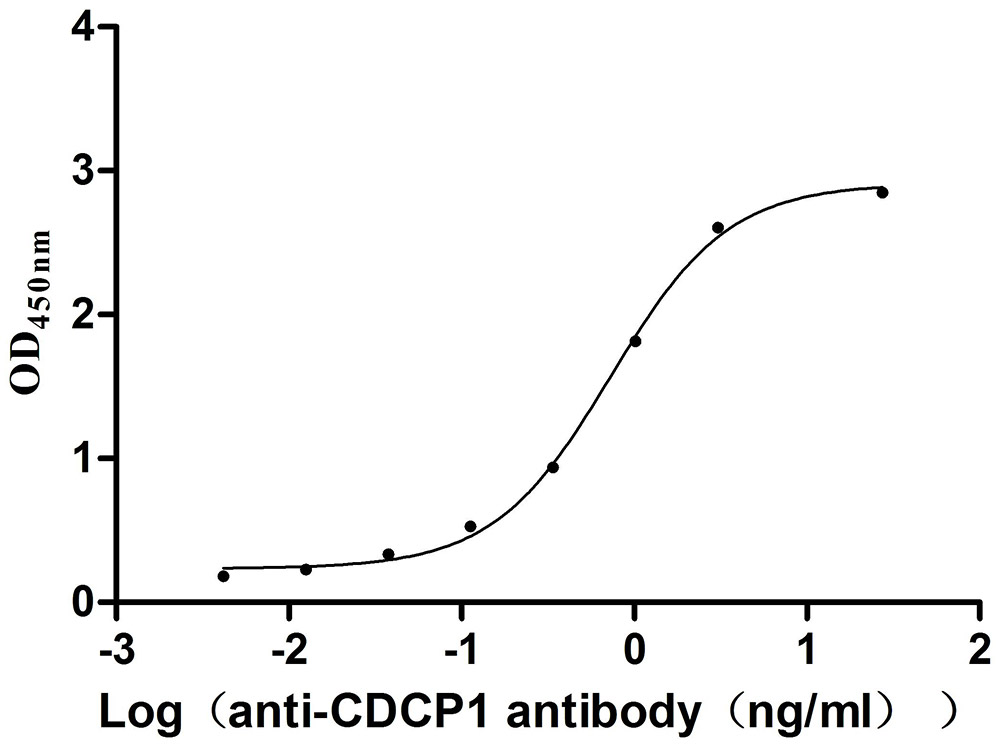
Recombinant Mouse CUB domain-containing protein 1 (Cdcp1), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
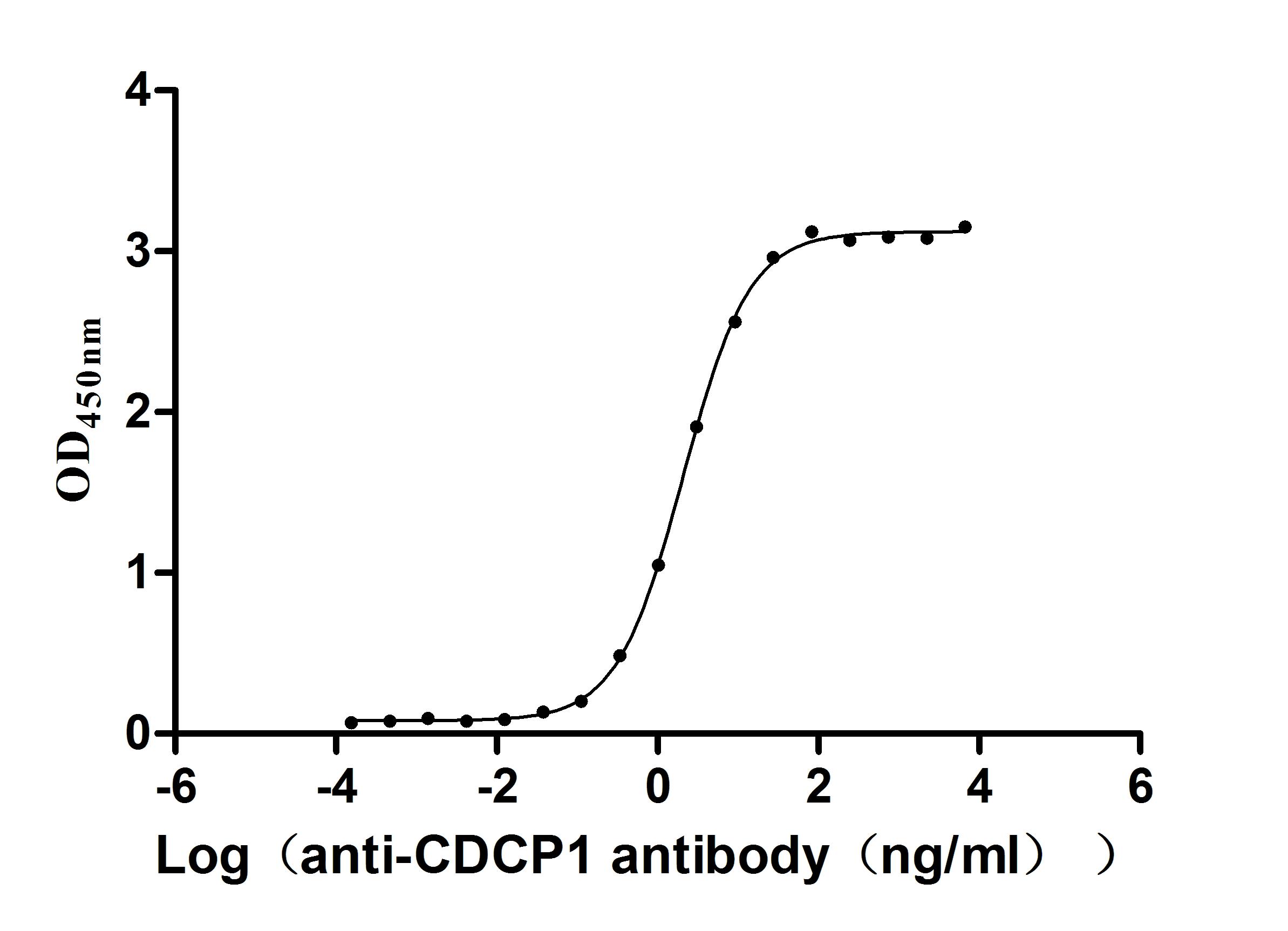
Recombinant Macaca fascicularis CUB domain containing protein 1 (CDCP1), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Macaca fascicularis C-type lectin domain family 4 member C(CLEC4C), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Human B- and T-lymphocyte attenuator(BTLA), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)


-AC1.jpg)