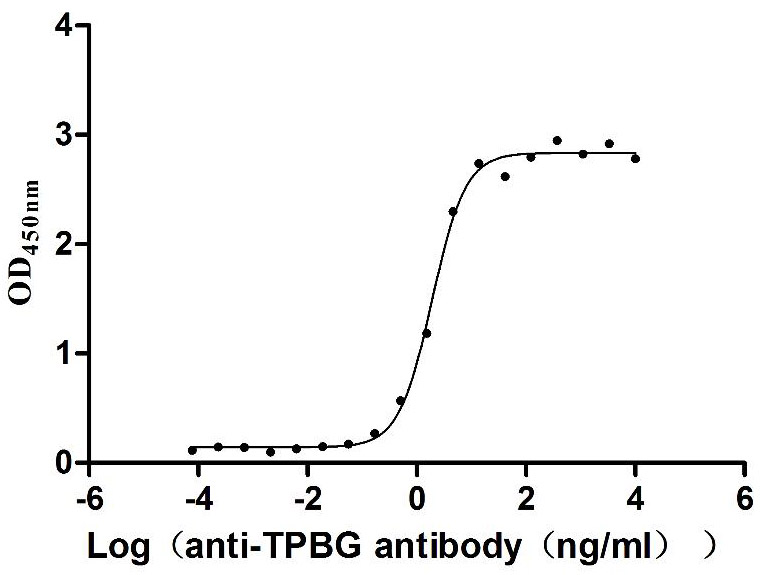
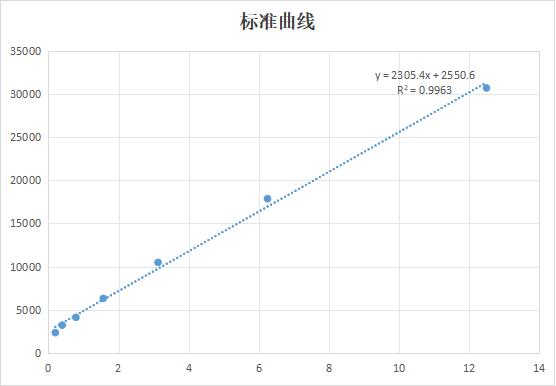
Recombinant Human Voltage-gated hydrogen channel 1 (HVCN1)
-
货号:CSB-CF846602HU
-
规格:
-
来源:in vitro E.coli expression system
-
其他:
产品详情
-
基因名:HVCN1
-
Uniprot No.:
-
别名:HVCN1; VSOP; UNQ578/PRO1140; Voltage-gated hydrogen channel 1; Hydrogen voltage-gated channel 1; HV1; Voltage sensor domain-only protein
-
种属:Homo sapiens (Human)
-
蛋白长度:full length protein
-
表达区域:1-273
-
氨基酸序列MATWDEKAVTRRAKVAPAERMSKFLRHFTVVGDDYHAWNINYKKWENEEEEEEEEQPPPT PVSGEEGRAAAPDVAPAPGPAPRAPLDFRGMLRKLFSSHRFQVIIICLVVLDALLVLAEL ILDLKIIQPDKNNYAAMVFHYMSITILVFFMMEIIFKLFVFRLEFFHHKFEILDAVVVVV SFILDIVLLFQEHQFEALGLLILLRLWRVARIINGIIISVKTRSERQLLRLKQMNVQLAA KIQHLEFSCSEKEQEIERLNKLLRQHGLLGEVN
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal 10xHis-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:Lyophilized from Tris/PBS-based buffer, 6% Trehalose, pH 8.0
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:Mediates the voltage-dependent proton permeability of excitable membranes. Forms a proton-selective channel through which protons may pass in accordance with their electrochemical gradient. Proton efflux, accompanied by membrane depolarization, facilitates acute production of reactive oxygen species in phagocytosis.
-
基因功能参考文献:
- The s now report that R1H mutation is sufficient to reconstitute resting-state H(+) 'shuttle' conductance in Hv1 without abrogating the intrinsic 'aqueous' H(+) conductance. PMID: 27572256
- We suggest that cleavage and heterodimerization of Hv1 represents an adaptation to the specific requirements of pH control in sperm. PMID: 27859356
- Inhibition of Hv1 channels by Zn(2+). PMID: 28013412
- CREBBP mutations were associated with inferior progression-free survival (PFS), whereas mutations in previously unreported HVCN1, a voltage-gated proton channel-encoding gene and B-cell receptor signaling modulator, were associated with improved PFS. PMID: 28064239
- Trp207 is crucial for slow channel opening, highly temperature-dependent gating kinetics, proton selectivity, and DeltapH-dependent gating. PMID: 26458876
- Our data demonstrated that the expression of Hv1 in pancreatic islet beta-cells regulates insulin secretion through regulating Ca(2+) homeostasis. PMID: 26559003
- The main properties of the voltage-gated proton channel (HV1) are described in this review, along with what is known about how the channel protein structure accomplishes its functions. [review] PMID: 25964989
- Hv1 activity displays hysteresis PMID: 25296308
- A shorter isoform of HVCN1 with enhanced gating is specifically enriched in malignant B cells. PMID: 25425665
- Divalent metal binding causes a conformational change in human the Hv1 c-terminal domain. PMID: 24867409
- analysis of of the C-terminal domain of voltage-gated proton channel HV1 and the thermodynamic characteristics of Zn(2) binding to this domain PMID: 25446125
- Salt bridge networks and the hydrophobic plug function as the gate in Hv1 channels; outward movement of the fourth transmembrane segment leads to the opening of this gate. PMID: 24379371
- Results suggest that Hv1 may be used as a potential biomarker for diagnosis and prognosis of colorectal carcinoma, and a potential target for anticancer drugs in colorectal cancer therapy. PMID: 23940591
- inhibition of Hv1 activity via Zn(2+) ions can effectively retard the cancer growth and suppress the cancer metastasis by the decrease of proton extrusion and the down-regulation of gelatinase activity PMID: 23891691
- In the Hv1 voltage-gated channel a highly conserved phenylalanine is found in the charge transfer center. PMID: 23352164
- Two conformational changes are detected in Hv1 channels that are involved in channel opening. PMID: 23352165
- inhibition of Hv1 function via knockdown of Hv1 expression can effectively retard cancer growth PMID: 22367212
- Hv1 channels maintain a physiological membrane potential during the respiratory burst of neutrophils by providing a compensating charge for the electrons transferred by NOX2 from NADPH to superoxide. PMID: 22056415
- Hv1-dependent reactive oxygen species production is responsible for a substantial fraction of brain damage at early time points after ischemic stroke. PMID: 22388960
- Interactions with two of the S4 arginines and the formation of a well defined hydrophobic gap in the center of the Hv1 are key to the formation of a robust water wire. PMID: 21843503
- identification of aspartate 112 as a crucial component of the selectivity filter of H(V)1 PMID: 22020278
- these results strongly suggest that Hv1 regulates breast cancer intracellular pH and exacerbates the migratory ability of metastatic cells. PMID: 21821008
- Data demonstrated that a massive reduction in H(v)1 expression can limit the Nox2 mediated superoxide production of PLB-985 granulocytes. PMID: 21124855
- The HVCN1 H(+) channel mediates pH-regulated acid secretion by the airway epithelium. Apical HVCN1 represents a mechanism to acidify an alkaline airway surface liquid. PMID: 20548053
- Report on the interaction of HV1 proton channel protomers during ion channel gating. PMID: 20676047
- HVCN1 not only modulates signaling from the B-cell receptor following B-cell activation and histamine release from basophils, but also mediates pH-dependent activation of spermatozoa, as well as acid secretion by tracheal epithelium. [Review] PMID: 20961760
- Molecular dynamics simulations revealed water molecules in the central crevice of Hv1 model structures but not in homologous voltage-sensor domain (VSD) structures. PMID: 20543828
- In the 2.0 A structure of the C-terminal domain, the two monomers form a dimer via a parallel alpha-helical coiled-coil, in which one chloride ion binds with the Neta atom of Arg(264). PMID: 20147290
- Identification of Thr29 as a critical phosphorylation site that activates the human proton channel Hvcn1 in leukocytes PMID: 20037153
- Since Hv1 specifically mediates proton efflux, it is likely to be the long-sought molecule that controls male fertility by mediating intracellular alkalinization of human sperm PMID: 20144758
- Hv1 channels truncated just downstream of R2 in the S4 segment retain most channel properties. PMID: 20018719
- data presented here identify H(v)1 as a long-sought voltage-gated H+ channel and establish H(v)1 as the founding member of a family of mammalian VSD proteins PMID: 16554753
- We cloned a new B cell-specific tetraspanning (BTS) membrane molecule PMID: 17948262
- Hv1 forms a dimer in the membrane;its regions that are close to the dimer interface were defined. PMID: 18509058
- The Hv1 channel voltage sensor domain by itself supports H(+) flux. PMID: 19233200
- The C-terminal domain of the human voltage-gated proton channel Hv1 (C-Hv1) was overexpressed in Escherichia coli, purified and crystallized using the hanging-drop vapour-diffusion method. PMID: 19255483
显示更多
收起更多
-
亚细胞定位:Membrane; Multi-pass membrane protein. Cell membrane; Multi-pass membrane protein. Note=Detected mainly at intracellular membranes upon overexpression in HeLa cells (PuMed:20147290), but not in other cell types.
-
蛋白家族:Hydrogen channel family
-
组织特异性:Enriched in immune tissues, such as lymph nodes, B-lymphocytes, monocytes and spleen.
-
数据库链接:
HGNC: 28240
OMIM: 611227
KEGG: hsa:84329
STRING: 9606.ENSP00000242607
UniGene: Hs.211511
Most popular with customers
-
Recombinant Human Heat-stable enterotoxin receptor (GUCY2C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human IGF-like family receptor 1 (IGFLR1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Mouse Transthyretin (Ttr) (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Human Cannabinoid receptor 1 (CNR1)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Mouse Complement component C1q receptor (Cd93), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Macaca fascicularis Trophoblast glycoprotein (TPBG), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Human Urokinase-type plasminogen activator(PLAU) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)