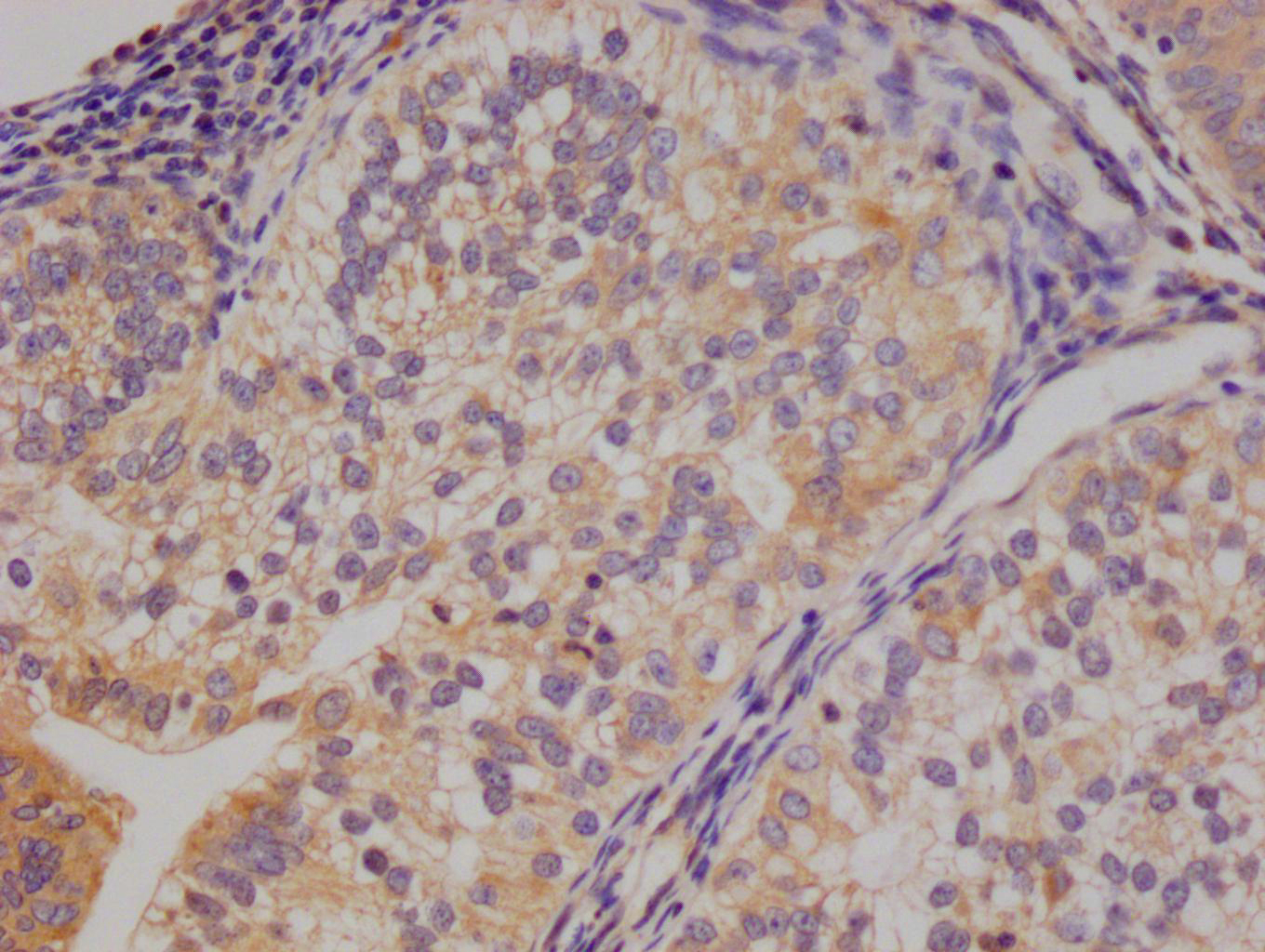
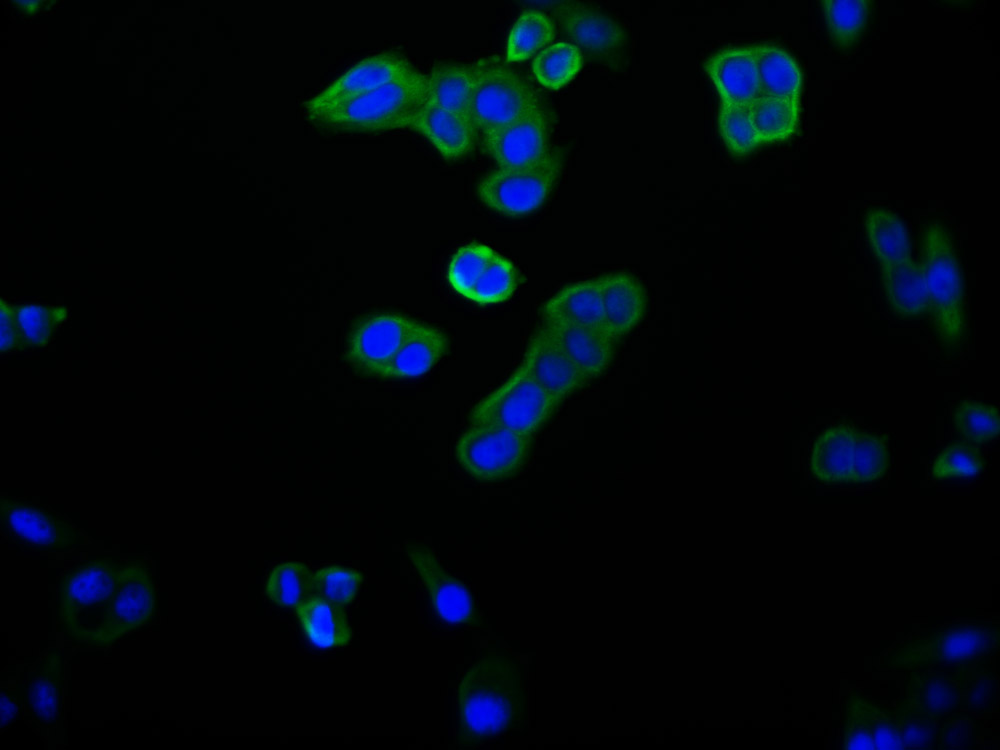
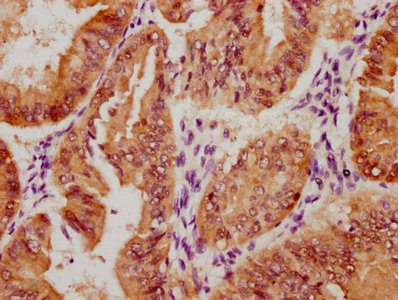
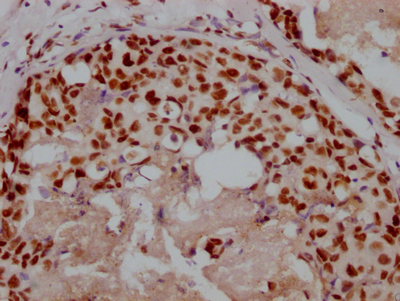
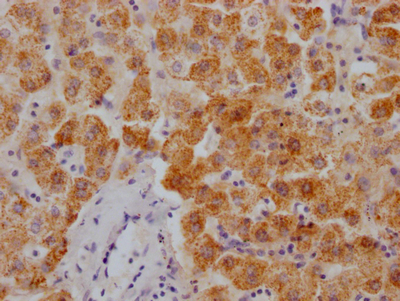
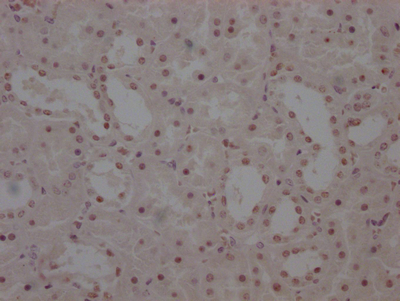
MYBPC3 Antibody
-
中文名称:MYBPC3兔多克隆抗体
-
货号:CSB-PA010534
-
规格:¥880
-
其他:
产品详情
-
Uniprot No.:Q14896
-
基因名:MYBPC3
-
别名:C protein cardiac muscle isoform antibody; C-protein antibody; cardiac muscle isoform antibody; Cardiac MyBP C antibody; Cardiac MyBP-C antibody; Cardiac myosin binding protein C antibody; cardiac-type antibody; CMH4 antibody; FHC antibody; MYBP C antibody; MYBPC antibody; MYBPC3 antibody; Myosin binding protein C cardiac antibody; Myosin binding protein C cardiac-type antibody; Myosin-binding protein C antibody; myosin-binding protein C cardiac type antibody; MYPC3_HUMAN antibody
-
宿主:Rabbit
-
反应种属:Human
-
免疫原:Synthesized peptide derived from the Internal region of Human MYBPC3.
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
-
抗体亚型:IgG
-
纯化方式:The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
-
产品提供形式:Liquid
-
应用范围:IHC, ELISA
-
推荐稀释比:
Application Recommended Dilution IHC 1:100-1:300 ELISA 1:10000 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Thick filament-associated protein located in the crossbridge region of vertebrate striated muscle a bands. In vitro it binds MHC, F-actin and native thin filaments, and modifies the activity of actin-activated myosin ATPase. It may modulate muscle contraction or may play a more structural role.
-
基因功能参考文献:
- Of the 52 hypertrophic cardiomyopathy patients 11 (21.2%) had MYBPC3 variants. PMID: 29386531
- MYBPC3 does not seem to play a role in anthracycline induced cardiotoxicity. PMID: 29716714
- Mutation in MYBPC3 was identified as Restrictive Cardiomyopathy - causing mutation. PMID: 27339502
- pathogenic gene mutations in LMNA and MYBPC3 alter RNA splicing and may have a role in heart disease PMID: 28679633
- MYBPC3 mutation carriers had a high frequency of ventricular arrhythmia and syncope. An absence of family history of sudden death (SD) and past history of syncope are useful prognostic factors in patients with hypertrophic cardiomyopathy. MYH7 and MYBPC3 mutations did not significantly influence prognosis compared to non-carriers. The patients with the MYBPC3 mutation should be closely followed for the possibility of SD. PMID: 27885498
- Replacement Fibrosis was most abundantly present in the hearts with a MYBPC3 mutation that first presented with a Hypertrophic Cardiomyopathy. PMID: 28365402
- Mutations in the gene MYBPC3 coding for cardiac myosin-binding protein-C (cMyBP-C), a multi-domain protein, are the most common cause of hypertrophic cardiomyopathy ..This molecular insight suggests that key HCM-causing mutations might significantly modify the native affinity required for the assembly of the domains in cMyBP-C, which is essential for normal cardiac function. PMID: 27267291
- Five of the 19 patients (26.3%) had either a pathogenic variant or a likely pathogenic variant in MYBPC3 (n=1), MYH7 (n=1), RYR2 (n=2), or TNNT2 (n=1). All five variants were missense variants that have been reported previously in patients with channelopathies or cardiomyopathies PMID: 28202948
- Double heterozygotes for mutations in DSP and MYBPC3 showed a variable clinical presentation of arrhythmogenic cardiomyopathy and hypertrophic cardiomyopathy. PMID: 28699631
- Data provide evidence that MYBPC3 mutations constitute the preeminent cause of hypertrophic cardiomyopathy (HCM) and that they are phenotypically indistinguishable from HCM caused by MYH7 mutations. PMID: 29121657
- In children and young adults, a 2-parameter 12-lead A-ECG score is retrospectively significantly more sensitive and specific than pooled, age-specific conventional ECG criteria for detecting MYBPC3-HCM and in distinguishing such patients from healthy controls, including endurance-trained athletes. PMID: 27061026
- mutations associated with a reduced super-relaxed state in hypertrophic cardiomyopathy PMID: 28658286
- Study showed that CACNB2 is a possible candidate hypertrophy-modifying gene contributing to disease variability of MYBPC3-associated familial hypertrophic cardiomyopathy PMID: 28614222
- The s demonstrate myosin tail (S2)-dependent functional regulation of actin-activated human beta-cardiac myosin ATPase. In addition, they show that both S2 and MyBP-C bind to S1 and that phosphorylation of either S1 or MyBP-C weakens these interactions. PMID: 28481356
- Our study supports that mutations in MYH7 and MYBPC3 should be the first focus of moleculargenetic analysis in HCM, and that mutations in TNNT2 have a low prevalence in Brazilian population. All mutations detected were missense mutations, whereas two mutations in MYH7 had not been described before. PMID: 27737317
- These findings point to the critical role of MYBPC3 during sarcomere assembly in cardiac myocyte differentiation and suggest developmental influences of MYBPC3 truncating mutations on the mature hypertrophic phenotype. PMID: 27620334
- The p.Pro108Alafs*9 mutation is associated with HCM, high penetrance, and disease onset in middle age PMID: 28029522
- Study shows lack of phenotypic differences between MYH7- and MYBPC3-associated hypertrophic cardiomyopathy when assessed by cardiac magnetic resonance imaging. PMID: 28193612
- MYBPC3 and MYH7 were the most common mutated genes, accounting for 27% of the total Hypertrophic Cardiomyopathy patients and 83% of the putative mutations in the main sarcomeric genes. PMID: 27574918
- MYBPC3 gene mutation is associated with Early-Onset Hypertrophic Cardiomyopathy. PMID: 27483260
- The results showed that MYBPC3 25-bp deletion polymorphism was significantly associated with elevated risk of left ventricular dysfunction (LVD), while TTN 18 bp I/D, TNNT2 5 bp I/D and myospryn K2906N polymorphisms did not show any significant association with LVD. PMID: 27350668
- we report a patient presenting with a complex phenotype consisting of severe, adult-onset, dilated cardiomyopathy, hearing loss and developmental delay, in which exome sequencing revealed two genetic variants that are inherited from a healthy mother: a variant, in MYBPC3, that is associated with hereditary cardiomyopathy. PMID: 27173948
- 5 out of 102 (4.9%) athletes carried mutations: a heterozygous MYH7 Glu935Lys mutation, a heterozygous MYBPC3 Arg160Trp mutation and another heterozygous MYBPC3 Thr1046Met mutation, all of which had been reported as HCM-associated mutations PMID: 26178432
- the phosphorylation pattern of sMyBP-C is differentially regulated in response to age and disease, suggesting that phosphorylation plays important roles in these processes. PMID: 26287277
- a significant role of MYBPC3 gene mutations in Hypertrophic cardiomyopathy(HCM) disease and can be used for pre-symptomatic diagnosis of at risk family members of affected individuals. PMID: 27348999
- Atrial fibrillation occurred in 74 patients with hypertrophic cardiomyopathy (31%), but with no difference among genotype groups (31% in MYBPC3, 37% in MYH7 and 18% in other genotypes, p = 0.15). PMID: 26869393
- The detection of MYBPC3 mutation, especially the PTC mutation and double-mutation, may serve as a molecular marker for clinical risk stratification of HCM. PMID: 26090888
- Demonstrate that MYBPC3 gene mutations, revealed by next-generation sequencing, were associated with familial and sporadic restrictive cardiomyopathy phenotype in patients. PMID: 26163040
- Data indicate that homozygous or compound heterozygous truncating pathogenic myosin binding protein C (MYBPC3) mutations cause severe neonatal cardiomyopathy with features of left ventricular noncompaction and septal defects. PMID: 25335496
- Case Report: double cMyBP-C mutation in a patient with end-stage hypertrophic cardiomyopathy. PMID: 25971843
- Mutations in the MYBPC3 and CASQ2 genes and six combinations between loci in the MYBPC3, MYH7 and CASQ2 genes were responsible for cardiomyopathy risk in a studied cohort. PMID: 25892673
- Although females with MYBPC3 mutations showed later onset of hypertrophic cardiomyopathy, female patients were more symptomatic at diagnosis and had more frequent heart failure events once they had developed hypertrophy. PMID: 25123604
- A founder MYBPC3 mutation results in HCM with a high risk of sudden death after the fourth decade of life PMID: 25740977
- Mutations in MYBPC3 are associated with cardiomyopathy. [Review] PMID: 26358504
- Characterization of the novel splicing mutations in cardiomyopathy genes MYBPC3 and TNNT2. PMID: 25849606
- Gene-specific severity of cardiac abnormalities may underlie differences in disease onset and suggests that early initiation of metabolic treatment may be beneficial, in particular, in myosin heavy chain (MYH7) mutation carriers PMID: 24835277
- These results show that the N-terminal region of MyBP-C stabilizes the ON state of thin filaments and the OFF state of thick filaments and lead to a novel hypothesis for the physiological role of MyBP-C in the regulation of cardiac contractility. PMID: 25512492
- This review summarizes evidence that phosphorylation of MyBP-C is a key regulator of cardiac force and contraction. PMID: 25552695
- at the cMyBP-C expression levels in hypertrophic cardiomyopathy patients, cross-bridge kinetics are preserved and that the depressed maximal force development is not explained by perturbation of cross-bridge kinetics PMID: 24186209
- A founder MYBPC3 mutation that arose >550 years ago is the predominant cause of hypertrophic cardiomyopathy in Iceland. PMID: 25078086
- These results demonstrate that MYBPC3 Val762Asp may be associated with unfavorable hypertrophic cardiomyopathy phenotypes PMID: 25281569
- A review of MYBPC3 mutations associated with hypertrophic cardiomyopathy. PMID: 24337823
- The structural features of the R502W mutation of myosin bindin protein C are described and discussed. PMID: 25058872
- In this review, we will address what is known of cMyBP-C's role as a regulator of contraction as well as its role in HCM[review] PMID: 24240729
- cMyBP-C is a key regulator of cardiac contractility. Although mutations in the gene encoding cMyBP-C are a leading cause of hypertrophic cardiomyopathy, little is known about the molecular mechanisms underlying the disease process[review] PMID: 24327208
- Change in the ability of cMyBP-C to bind cardiac actin modified filaments might contribute to the development of disease. PMID: 24736382
- G263X mutation of MYBPC3 was found in 7 patients with hypertrophic cardiomyopathy in Asturias, Spain. PMID: 23870641
- Hypertrophic cardiomyopathy patients with MYBPC3 mutations have a specific miRNA expression profile. PMID: 24083979
- N-terminal fragment of cardiac myosin-binding protein C (cMyBP-C) impairs myofilament function in human myocardium PMID: 24509847
- cMyBP-C is released in the blood rapidly after cardiac damage and therefore has the potential to positively mark the onset of myocardial infarction. PMID: 24337456
显示更多
收起更多
-
相关疾病:Cardiomyopathy, familial hypertrophic 4 (CMH4); Cardiomyopathy, dilated 1MM (CMD1MM); Left ventricular non-compaction 10 (LVNC10)
-
蛋白家族:Immunoglobulin superfamily, MyBP family
-
数据库链接:
HGNC: 7551
OMIM: 115197
KEGG: hsa:4607
STRING: 9606.ENSP00000442795
UniGene: Hs.524906
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-