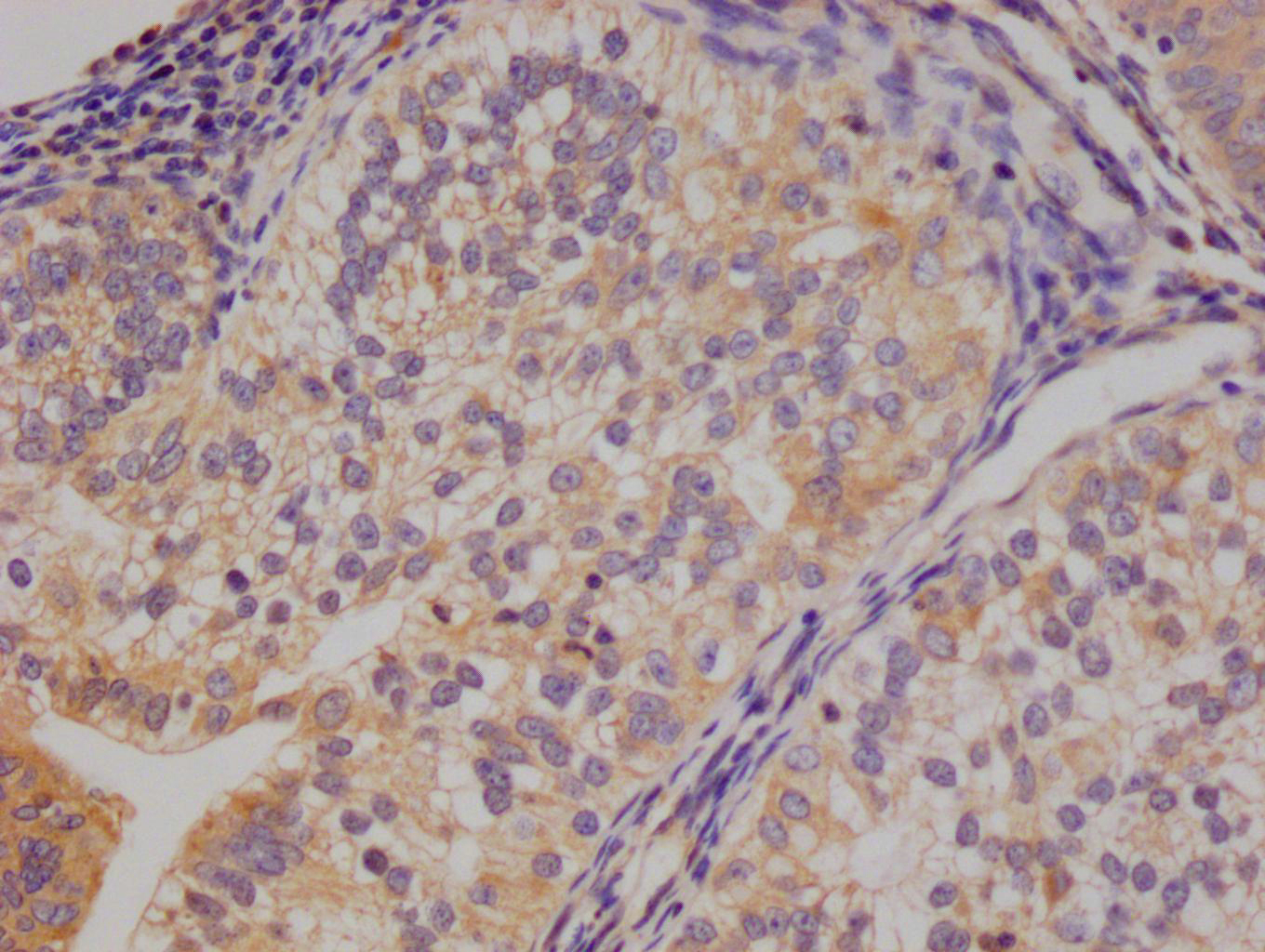
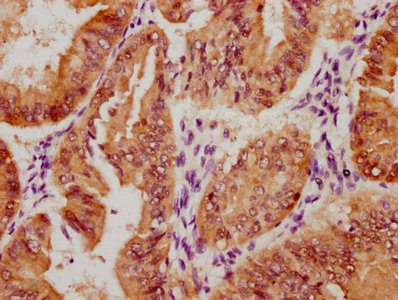
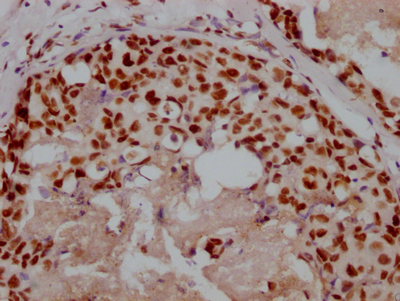
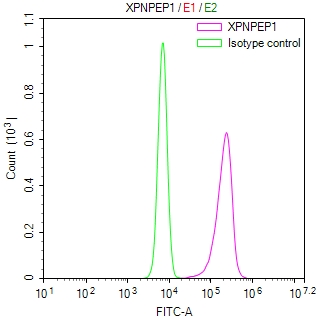
SIRT2 Antibody
-
中文名称:SIRT2兔多克隆抗体
-
货号:CSB-PA088519
-
规格:¥1100
-
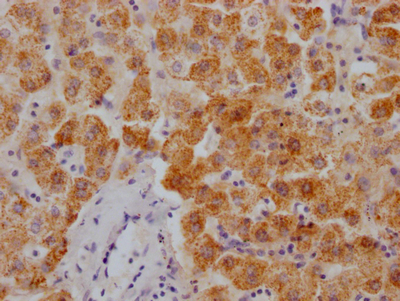
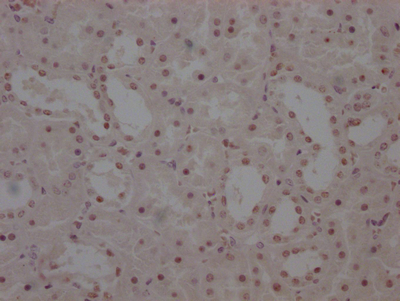
图片:
-
其他:
产品详情
-
Uniprot No.:Q8IXJ6
-
基因名:SIRT2
-
别名:FLJ35621 antibody; FLJ37491 antibody; NAD dependent deacetylase sirtuin 2 antibody; NAD dependent protein deacetylase sirtuin 2 antibody; NAD-dependent deacetylase sirtuin-2 antibody; NAD-dependent protein deacetylase sirtuin-2 antibody; Regulatory protein SIR2 homolog 2 antibody; Silencing information regulator 2 like antibody; Silent information regulator 2 antibody; SIR2 antibody; SIR2 like protein 2 antibody; Sir2 related protein type 2 antibody; SIR2, S. cerevisiae, homolog-loke 2 antibody; SIR2-like protein 2 antibody; SIR2L antibody; SIR2L2 antibody; SIRT2 antibody; SIRT2_HUMAN antibody; Sirtuin (silent mating type information regulation 2 homolog) 2 (S.cerevisiae) antibody; Sirtuin 2 antibody; Sirtuin type 2 antibody
-
宿主:Rabbit
-
反应种属:Human,Mouse,Rat
-
免疫原:Fusion protein of Human SIRT2
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
-
抗体亚型:IgG
-
纯化方式:Antigen affinity purification
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:-20°C, pH7.4 PBS, 0.05% NaN3, 40% Glycerol
-
产品提供形式:Liquid
-
应用范围:ELISA,WB
-
推荐稀释比:
Application Recommended Dilution ELISA 1:1000-1:2000 WB 1:200-1:1000 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:NAD-dependent protein deacetylase, which deacetylates internal lysines on histone and alpha-tubulin as well as many other proteins such as key transcription factors. Participates in the modulation of multiple and diverse biological processes such as cell cycle control, genomic integrity, microtubule dynamics, cell differentiation, metabolic networks, and autophagy. Plays a major role in the control of cell cycle progression and genomic stability. Functions in the antephase checkpoint preventing precocious mitotic entry in response to microtubule stress agents, and hence allowing proper inheritance of chromosomes. Positively regulates the anaphase promoting complex/cyclosome (APC/C) ubiquitin ligase complex activity by deacetylating CDC20 and FZR1, then allowing progression through mitosis. Associates both with chromatin at transcriptional start sites (TSSs) and enhancers of active genes. Plays a role in cell cycle and chromatin compaction through epigenetic modulation of the regulation of histone H4 'Lys-20' methylation (H4K20me1) during early mitosis. Specifically deacetylates histone H4 at 'Lys-16' (H4K16ac) between the G2/M transition and metaphase enabling H4K20me1 deposition by KMT5A leading to ulterior levels of H4K20me2 and H4K20me3 deposition throughout cell cycle, and mitotic S-phase progression. Deacetylates KMT5A modulating KMT5A chromatin localization during the mitotic stress response. Deacetylates also histone H3 at 'Lys-57' (H3K56ac) during the mitotic G2/M transition. Upon bacterium Listeria monocytogenes infection, deacetylates 'Lys-18' of histone H3 in a receptor tyrosine kinase MET- and PI3K/Akt-dependent manner, thereby inhibiting transcriptional activity and promoting late stages of listeria infection. During oocyte meiosis progression, may deacetylate histone H4 at 'Lys-16' (H4K16ac) and alpha-tubulin, regulating spindle assembly and chromosome alignment by influencing microtubule dynamics and kinetochore function. Deacetylates histone H4 at 'Lys-16' (H4K16ac) at the VEGFA promoter and thereby contributes to regulate expression of VEGFA, a key regulator of angiogenesis. Deacetylates alpha-tubulin at 'Lys-40' and hence controls neuronal motility, oligodendroglial cell arbor projection processes and proliferation of non-neuronal cells. Phosphorylation at Ser-368 by a G1/S-specific cyclin E-CDK2 complex inactivates SIRT2-mediated alpha-tubulin deacetylation, negatively regulating cell adhesion, cell migration and neurite outgrowth during neuronal differentiation. Deacetylates PARD3 and participates in the regulation of Schwann cell peripheral myelination formation during early postnatal development and during postinjury remyelination. Involved in several cellular metabolic pathways. Plays a role in the regulation of blood glucose homeostasis by deacetylating and stabilizing phosphoenolpyruvate carboxykinase PCK1 activity in response to low nutrient availability. Acts as a key regulator in the pentose phosphate pathway (PPP) by deacetylating and activating the glucose-6-phosphate G6PD enzyme, and therefore, stimulates the production of cytosolic NADPH to counteract oxidative damage. Maintains energy homeostasis in response to nutrient deprivation as well as energy expenditure by inhibiting adipogenesis and promoting lipolysis. Attenuates adipocyte differentiation by deacetylating and promoting FOXO1 interaction to PPARG and subsequent repression of PPARG-dependent transcriptional activity. Plays a role in the regulation of lysosome-mediated degradation of protein aggregates by autophagy in neuronal cells. Deacetylates FOXO1 in response to oxidative stress or serum deprivation, thereby negatively regulating FOXO1-mediated autophagy. Deacetylates a broad range of transcription factors and co-regulators regulating target gene expression. Deacetylates transcriptional factor FOXO3 stimulating the ubiquitin ligase SCF(SKP2)-mediated FOXO3 ubiquitination and degradation. Deacetylates HIF1A and therefore promotes HIF1A degradation and inhibition of HIF1A transcriptional activity in tumor cells in response to hypoxia. Deacetylates RELA in the cytoplasm inhibiting NF-kappaB-dependent transcription activation upon TNF-alpha stimulation. Inhibits transcriptional activation by deacetylating p53/TP53 and EP300. Deacetylates also EIF5A. Functions as a negative regulator on oxidative stress-tolerance in response to anoxia-reoxygenation conditions. Plays a role as tumor suppressor.; Deacetylates EP300, alpha-tubulin and histone H3 and H4.; Deacetylates EP300, alpha-tubulin and histone H3 and H4.; Lacks deacetylation activity.
-
基因功能参考文献:
- Our data provide strong evidence that sirtuin-2 controls the functional ability of the autophagic system through acetylation and highlight the association between mitochondrial metabolism and neurodegeneration in sporadic Parkinson's disease. PMID: 28168426
- Data found that SIRT2 is a novel deacetylase of HSP90, enhances its ubiquitination-mediated proteasomal degradation, and consequently down-regulates HSP90/LIMK1/cofilin-linked actin polymerization regulation pathway. The deacetylase activity of SIRT2 is required to reduce cell motility by regulating the stability of HSP90. These results demonstrate that SIRT2 functions as a tumor suppressor. PMID: 29908203
- we provide insight into the regulation of SIRT2 on gastric cancer metabolism and metastasis. SIRT2 increased PEPCK1 protein levels and mitochondrial activity, as well as induced cell migration and invasion by activating the RAS/ERK/JNK/MMP-9 pathway PMID: 29925042
- MiR150 plays an important role in the development of lung cancer by serving as an oncogene via the SIRT2/JMJD2A signaling pathway. PMID: 29901178
- Studied association of SIRT2 and p53/NF-kB p65 signal pathways in preventing high glucose-induced vascular endothelial cell injury. Results demonstrated that SIRT2 overexpression is associated with deacetylation of p53 and NF-kB p65, which inhibits the high glucose induced apoptosis and vascular endothelial cell inflammation response. PMID: 29189925
- The s report that one of the K-Ras splice variants, K-Ras4a, is subject to lysine fatty acylation, a previously under-studied protein post-translational modification. Sirtuin 2 (SIRT2), one of the mammalian nicotinamide adenine dinucleotide (NAD)-dependent lysine deacylases, catalyzes the removal of fatty acylation from K-Ras4a. PMID: 29239724
- Low SIRT2 expression is associated with recurrence in prostate cancer. PMID: 29262808
- SIRT2 participates in the activation of fibroblasts and tubulointerstitial fibrosis, which is mediated via regulation of the MDM2 pathway, and the downregulation of SIRT2. PMID: 29614506
- Findings suggested that the DNA sequence variants may increase SIRT2 gene promoter activity and SIRT2 levels, contributing to T2D development as a risk factor. PMID: 29371109
- Chemical inhibitors against SIRT2 suppress G6PD activity, leading to reduced cell proliferation of leukaemia cells, but not normal hematopoietic stem and progenitor cells. Importantly, SIRT2 is overexpressed in clinical acute myeloid leukaemia samples, while K403 acetylation is downregulated and G6PD catalytic activity is increased comparing to that of normal control. PMID: 27586085
- SIRT2 may have a role in unfavorable prognosis of acute myeloid leukemia PMID: 27291931
- Data suggest that inhibition of sirtuin 1 and sirtuin 2 in hepatocellular carcinoma cells (a) impairs cell survival and cell migration and (b) down-regulates expression of P-glycoprotein and MRP3 (ATP binding cassette subfamily C member 3). PMID: 29545174
- SIRT2 inhibition may improve microtubule assembly thus representing a valid approach as disease-modifying therapy for Alzheimer's disease. PMID: 27311773
- Data show that single nucleotide polymorphism rs2015C in sirtuin 2 protein (SIRT2) gene 3'-UTR was significantly associated with increased risk of colorectal cancer (CRC). PMID: 28514749
- s show that SIRT2 is downregulated in insulin-resistant hepatocytes and livers, and this was accompanied by increased generation of reactive oxygen species, activation of stress-sensitive ERK1/2 kinase, and mitochondrial dysfunction. PMID: 28973648
- The SIRT2 functions as a mitochondrial sirtuin, as well as a regulator of autophagy/mitophagy to maintain mitochondrial biology, thus facilitating cell survival. PMID: 27460777
- increased expression of SRF that was observed in the aged heart may affect SIRT2 gene expression and contribute to altered metabolic status in senescence PMID: 29267359
- SIRT2 and RIPK1 were localized to the syncytiotrophoblast, villous leukocytes and vasculature in all preterm placentas. A significant reduction in SIRT2 protein expression in both preeclampsia and fetal growth restricted placentas was identified. Immunofluorescence identified both SIRT2 and RIPK1 in the cytotrophoblast cytoplasm. PMID: 28292463
- mutations in sirtuin2 increase the stability of the conserved catalytic sirtuin domain, thereby increasing the catalytic efficiency of the mutant enzymes. PMID: 28273448
- targeting SIRT2 may be a rational strategy for diminishing Slug abundance and its associated malignant traits in basal-like breast cancer. PMID: 27783945
- BEX4 overexpression causes an imbalance between TUB acetylation and deacetylation by SIRT2 inhibition and induces oncogenic aneuploidy transformation. PMID: 27512957
- SIRT2 maintains cellular iron levels by binding to and deacetylating nuclear factor erythroid-derived 2-related factor 2 (NRF2) on lysines 506 and 508, leading to a reduction in total and nuclear NRF2 levels. PMID: 28287409
- Identify the miR-200c-SIRT2 axis as a key regulator of metabolic reprogramming (Warburg-like effect), via regulation of glycolytic enzymes, during human induced pluripotency and pluripotent stem cell function. PMID: 28436968
- Four novel heterozygous DNA sequence variants and five SNPs of sirt2 protein were found in both acute myocardial infarction patients and control with similar frequencies. PMID: 28445509
- ANKLE2 acetylation at K302 and phosphorylation at S662 are dynamically regulated throughout the cell cycle by SIRT2 and are essential for normal nuclear envelope reassembly. PMID: 27875273
- our findings suggest that the tumor suppressor activity of SIRT2 requires its ability to restrict the antioxidant activity of Prdx-1, thereby sensitizing breast cancer cells to reactive oxygen species -induced DNA damage and cell cytotoxicity PMID: 27503926
- Data suggest that SIRT2 exhibits tumor-suppressive function in which somatic mutations in SIRT2 contribute to genomic instability by impairing deacetylase activity of SIRT2 or diminishing its protein levels in the DNA-damage/repair response. PMID: 28461331
- Genetic manipulation of sirtuin 2 levels in vitro and in vivo modulates the levels of alpha-synuclein acetylation, its aggregation, and autophagy. PMID: 28257421
- the tissue from lymph node metastases appears to have a significant upregulation of SIRT2 relative to primary tumors across the nuclear, cytoplasmic, and whole cell data. PMID: 28166441
- Data show that SIRT2 interacts with multiple intracellular trafficking proteins and showed that majority of its interactions are of transient nature. Also, It confirms its colocalization with ER-Golgi intermediate compartment. PMID: 27503897
- The levels of SPOP significantly decreased, while the levels of SIRT2 significantly increased in non-small cell lung cancer (NSCLC) cell lines, compared to normal bronchial epithelial cell line and NSCLC specimens, compared to the paired non-tumor lung tissue. PMID: 28073696
- SIRT2 is a promising marker of cellular senescence at least in cells with wild type p53 status. PMID: 27229617
- The results suggest that Sirt2 plays a crucial role in neuronal differentiation via the ERK-CREB signaling pathway. PMID: 27838300
- Reduced SIRT2 expression during tumorigenesis failed to repress cyclindependent kinase 4 expression, which eventually led to accelerated cell proliferation. PMID: 28259910
- Sirt-2 is recruited to NF-kappaB target gene promoter via interaction with core histones. PMID: 27036868
- Study demonstrate that PRL is necessary for the survival of (retinal pigment epithelium) RPE under normal and advancing age conditions and, identified SIRT2 and TRPM2 as molecular targets for the antioxidant and antiapoptotic actions of PRL in the RPE. PMID: 27322457
- 4-oxo-2-nonenal reacts with histone lysine residues to form a new histone modification, gamma-oxononanoylation (Kgon). human Sirt2 catalyzes the removal of histone Kgon. PMID: 28103679
- Data suggest that SIRT2 may induce Skp2 deacetylation and subsequent degradation to abolish the effects of Skp2 on p27 to affect NSCLC cell growth. PMID: 26942878
- Data indicate that compared to non-neoplastic endometria (NNE), endometrial cancer (EC) showed SIRT7 mRNA overexpression, whereas SIRT1, SIRT2, SIRT4 and SIRT5 were underexpressed, and no significant differences were observed for SIRT3 and SIRT6. PMID: 26701732
- ATRIP deacetylation by SIRT2 promotes ATR-ATRIP binding to replication protein A-single-stranded DNA to drive ATR activation and thus facilitate recovery from replication stress. PMID: 26854234
- The implication of the overall nonspecificity of SIRT1 and SIRT2 on the nucleosome suggests that these sirtuin enzymes have an adaptive nature, harnessing an ability to respond to various cellular situations, rather than an enzyme specifically designed for a particular task or function. PMID: 26820517
- microseed matrix seeding (MMS) was used to obtain crystals of human Sirt3 in its apo form and of human Sirt2 in complex with ADP ribose (ADPR). PMID: 26625292
- Use of a pan-sirtuin inhibitor and shRNA-mediated protein knockdown led us to uncover a role for the NAD(+)-dependent family of sirtuins, and in particular for SIRT2 and SIRT5, in the regulation of the necroptotic cell death program PMID: 26001219
- Two polymorphisms,SIRT2-rs45592833 G/T and DRD2-rs6276 A/G, provided a significant association with human longevity. PMID: 25934993
- Emerging Role of Sirtuin 2 in the Regulation of Mammalian Metabolism PMID: 26538315
- Under hypoxic conditions, SIRT2 inhibition increased the ubiquitination of HIF-1alpha in a VHL-dependent manner, leading to the degradation of HIF-1alpha via a proteasomal pathway. PMID: 26808575
- Regulation of protein acetylation by SIRT2 plays a central role in platelet function. The effects of SIRT2 are mediated in part by the acetylation and inhibition of Akt. PMID: 25960087
- high-resolution structures of human Sirt2 in complex with highly selective drug-like inhibitors that show a unique inhibitory mechanism, are presented. PMID: 25672491
- Expression of sirtuin 1 and 2 is associated with poor prognosis in non-small cell lung cancer patients PMID: 25915617
- In conclusion our data reveal that resveratrol induced premature senescence is associated with SIRT1 and SIRT2 down regulation in human dermal fibroblasts PMID: 25924011
显示更多
收起更多
-
亚细胞定位:Nucleus. Cytoplasm, perinuclear region. Cytoplasm. Cytoplasm, cytoskeleton. Cytoplasm, cytoskeleton, microtubule organizing center, centrosome. Cytoplasm, cytoskeleton, microtubule organizing center, centrosome, centriole. Cytoplasm, cytoskeleton, spindle. Midbody. Chromosome. Perikaryon. Cell projection. Cell projection, growth cone. Myelin membrane.; [Isoform 1]: Cytoplasm. Nucleus. Note=Predominantly localized in the cytoplasmic.; [Isoform 2]: Cytoplasm. Nucleus. Note=Predominantly localized in the cytoplasmic.; [Isoform 5]: Cytoplasm. Nucleus. Note=Predominantly localized in the nucleus.
-
蛋白家族:Sirtuin family, Class I subfamily
-
组织特异性:Isoform 1 is expressed in heart, liver and skeletal muscle, weakly expressed in the cortex. Isoform 2 is strongly expressed in the cortex, weakly expressed in heart and liver. Weakly expressed in several malignancies including breast, liver, brain, kidney
-
数据库链接:
HGNC: 10886
OMIM: 604480
KEGG: hsa:22933
STRING: 9606.ENSP00000249396
UniGene: Hs.466693
Most popular with customers
-
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-
-