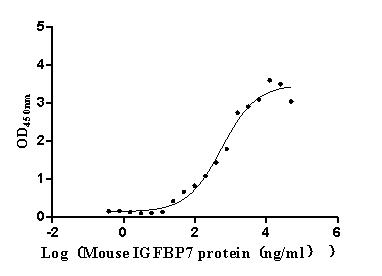
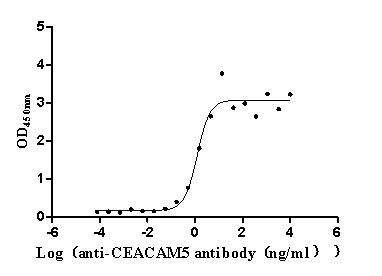
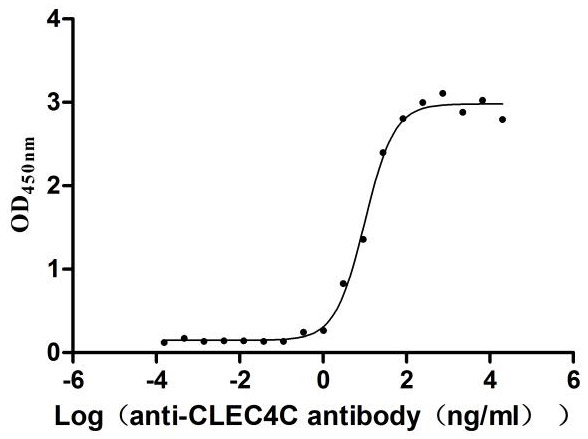
Recombinant Human CDGSH iron-sulfur domain-containing protein 1 (CISD1), partial
-
中文名称:Recombinant Human CDGSH iron-sulfur domain-containing protein 1(CISD1),partial,Yeast
-
货号:CSB-YP868365HU
-
规格:
-
来源:Yeast
-
其他:
-
中文名称:Recombinant Human CDGSH iron-sulfur domain-containing protein 1(CISD1),partial,Yeast
-
货号:CSB-EP868365HU
-
规格:
-
来源:E.coli
-
其他:
-
中文名称:Recombinant Human CDGSH iron-sulfur domain-containing protein 1(CISD1),partial,Yeast
-
货号:CSB-EP868365HU-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名称:Recombinant Human CDGSH iron-sulfur domain-containing protein 1(CISD1),partial,Yeast
-
货号:CSB-BP868365HU
-
规格:
-
来源:Baculovirus
-
其他:
-
中文名称:Recombinant Human CDGSH iron-sulfur domain-containing protein 1(CISD1),partial,Yeast
-
货号:CSB-MP868365HU
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:CISD1
-
Uniprot No.:
-
别名:AU043990; AW743335; C10orf70; CDGSH iron sulfur domain 1; CDGSH iron-sulfur domain-containing protein 1; CISD 1; CISD1; CISD1_HUMAN; D10Ertd214e; MDS029; MGC14684; MitoNEET; RGD1309529; ZCD1; Zinc finger CDGSH type domain 1
-
种属:Homo sapiens (Human)
-
蛋白长度:Partial
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Plays a key role in regulating maximal capacity for electron transport and oxidative phosphorylation. May be involved in Fe-S cluster shuttling and/or in redox reactions.
-
基因功能参考文献:
- The [2Fe-2S] clusters of mitoNEET are reduced via the formation of a transient complex that brings the [2Fe-2S] clusters of mitoNEET close to the redox-active [2Fe-2S] cluster of anamorsin. PMID: 28648056
- The results suggest that flavin nucleotides may act as electron shuttles to reduce the mitoNEET [2Fe-2S] clusters and regulate mitochondrial functions in human cells. PMID: 27923678
- Data suggest that, compared with oxygen, ubiquinone-2 is more efficient in oxidizing mitoNEET [2Fe-2S] clusters, suggesting that ubiquinone could be an intrinsic electron acceptor of reduced mitoNEET [2Fe-2S] clusters in mitochondrial outer membrane. PMID: 28461337
- CISD1 inhibits ferroptosis by protecting the cells against mitochondrial lipid peroxidation. PMID: 27510639
- the redox-sensing function of mNT is a key component of the cellular adaptive response to help stress-sensitive Fe-S proteins recover from oxidative injury. PMID: 26887944
- A possible role of CISD1 in obesity-associated dysfunctional adipogenesis in human visceral adipose tissue. PMID: 26692580
- Our results confirm the observation that mitoNEET is important in transferring the iron sulfur clusters to the cytosolic aconitase in living cells and the His-87 ligand in mitoNEET plays important role in this process. PMID: 26778000
- Glutathione reductase reduces mitochondrial protein mitoNEET [2Fe-2S] clusters. PMID: 25645953
- Studies indicate that NEET proteins are associated with diseases including cancer and diabetes. PMID: 25448035
- SNPs in three genes CYP26B1 rs2241057, CISD1 rs2251039, rs2590370, and TBX1 rs4819522 were involved in six potential pathways to influence serum prostate-specific antigen levels. PMID: 25168891
- In this review, we evaluate the current understanding regarding how mitoNEET regulates cellular bioenergetics as well as the structural requirements for drug compound association with mitoNEET PMID: 24814435
- MitoNEET governs a novel trafficking pathway to rebuild an Fe-S cluster into cytosolic aconitase/IRP1. PMID: 25012650
- pioglitazone may modulate the function of mitoNEET by blocking the thiol-mediated reduction of [2Fe-2S] clusters in the protein. PMID: 24403080
- The MitoNEET forms a covalent complex with GDH1 through disulfide bond formation and acts as an activator. PMID: 24295216
- Data show that the protein levels of NAF-1 (CISD2) and mNT (CISD1) are elevated in human epithelial breast cancer cells. PMID: 23959881
- a loop (L2) 20 A away from the metal center exerts allosteric control over the cluster binding domain and regulates multiple properties of the metal center. Mutagenesis of L2 results in significant shifts in the redox potential of the [2Fe-2S] cluster. PMID: 23271805
- NADPH can regulate both mitoNEET [2Fe-2S] cluster levels in the cell as well as the ability of the protein to transfer [2Fe-2S] clusters to cytosolic or mitochondrial acceptors. PMID: 22351774
- These findings suggest a likely role for mNT in [2Fe-2S] and/or iron transfer to acceptor proteins. PMID: 21788481
- crystal structure of H87C mitoNEET was determined to 1.7 A resolution (R factor = 18%) to investigate the structural basis of the changes in the properties of the 2Fe-2S cluster PMID: 21636891
- The iron-sulfur cluster-containing protein mitoNEET interacts with two potentially redox active substances at the surface of mitochondria; mitoNEET forms complexes with resveratrol-3-sulfate, a primary metabolite of the natural product resveratrol. PMID: 21591687
- Results describe the discovery of potential mitoNEET ligand binding sites and novel ligands, and suggests the possibility for detailed structural studies of mitoNEET-ligand complexes. PMID: 21531159
- Results describe the folding landscape of mitoNEET, and uncover communication between distal regions of the protein. PMID: 21402934
- Reduced nicotinamide adenine dinucleotide phosphate (NADPH) can bind to homodimeric mitoNEET, influencing the stability of the [2Fe-2S] cluster that is bound within a loop region (Y71-H87) in each subunit. PMID: 20932062
- We use electron paramagnetic resonance spectroscopy to investigate the [2Fe-2S]cluster in mitoNEET. PMID: 20099820
- mito- NEET is an important iron-containing protein involved in the control of maximal mitochondrial respiratory rates PMID: 17376863
- Spectroscopic studies show that the 2Fe-2S cluster is coordinated by Cys-3 and His-1. The His ligand is shown to be involved in the observed pH lability of the cluster, indicating that loss of this ligand via protonation triggered release of the cluster. PMID: 17584744
- Crystal structure of mitoNEET reveals distinct groups of iron sulfur proteins. PMID: 17766439
- biophysical properties of mitoNEET suggest that it may participate in a redox-sensitive signaling and/or in Fe-S cluster transfer PMID: 17766440
- X-ray crystallographic studies show that mitoNEET dimer may interact with other proteins via the surface residues in close proximity to the [2Fe-2S] cluster PMID: 17905743
- A CISD1-GFP chimera was found to be located into mitochondria. PMID: 18047834
- The physiologically relevant acid ionization constant (pKa) of histidine residues makes histidine87 a likely candidate for modulating the lability of the metal cluster in mitoNEET. PMID: 19388667
- There is considerable flexibility in the position of the cytoplasmic tethering arms, resulting in two different conformations in the crystal structure of mitoNEET. PMID: 19574633
显示更多
收起更多
-
亚细胞定位:Mitochondrion outer membrane; Single-pass type III membrane protein.
-
蛋白家族:CISD protein family
-
组织特异性:Expression is reduced in cells derived from cystic fibrosis patients.
-
数据库链接:
HGNC: 30880
OMIM: 611932
KEGG: hsa:55847
STRING: 9606.ENSP00000363041
UniGene: Hs.370102
Most popular with customers
-
Recombinant Human T-cell antigen CD7 (CD7), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
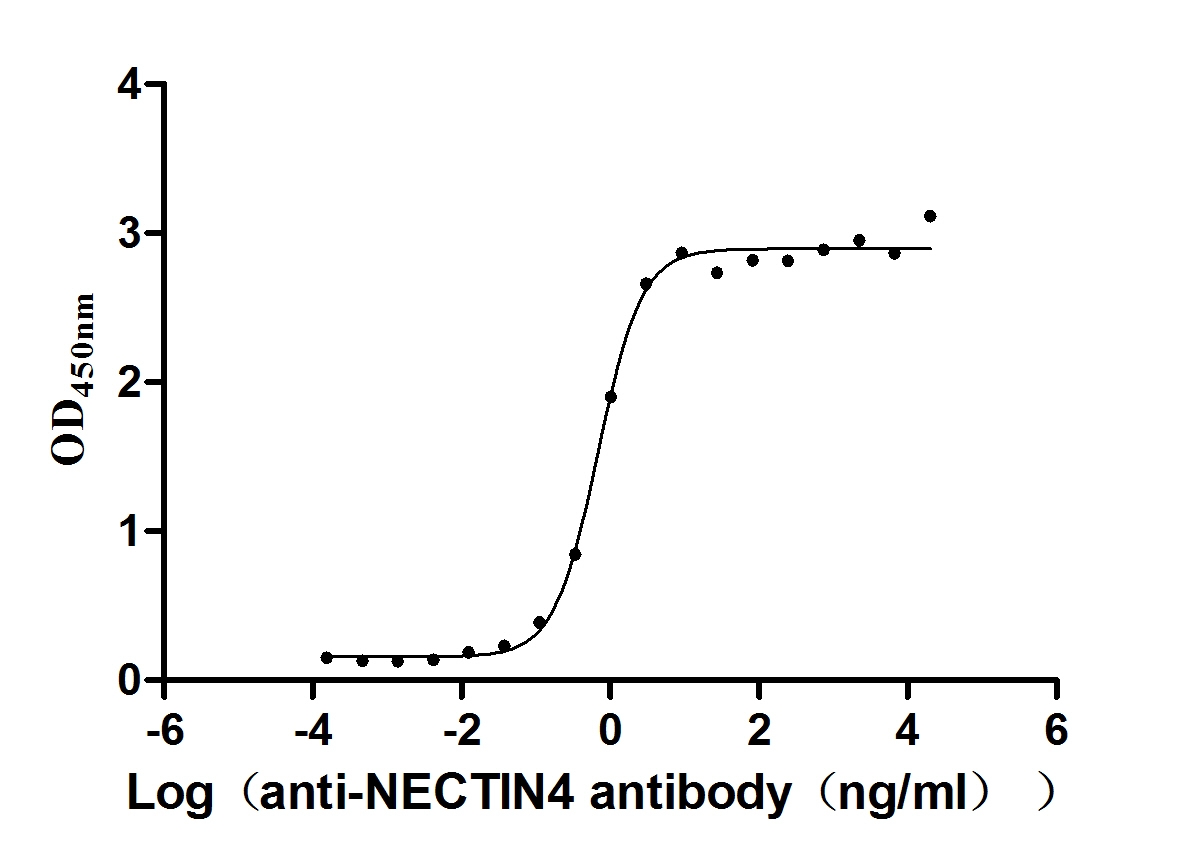
Recombinant Human Nectin-4 (NECTIN4), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
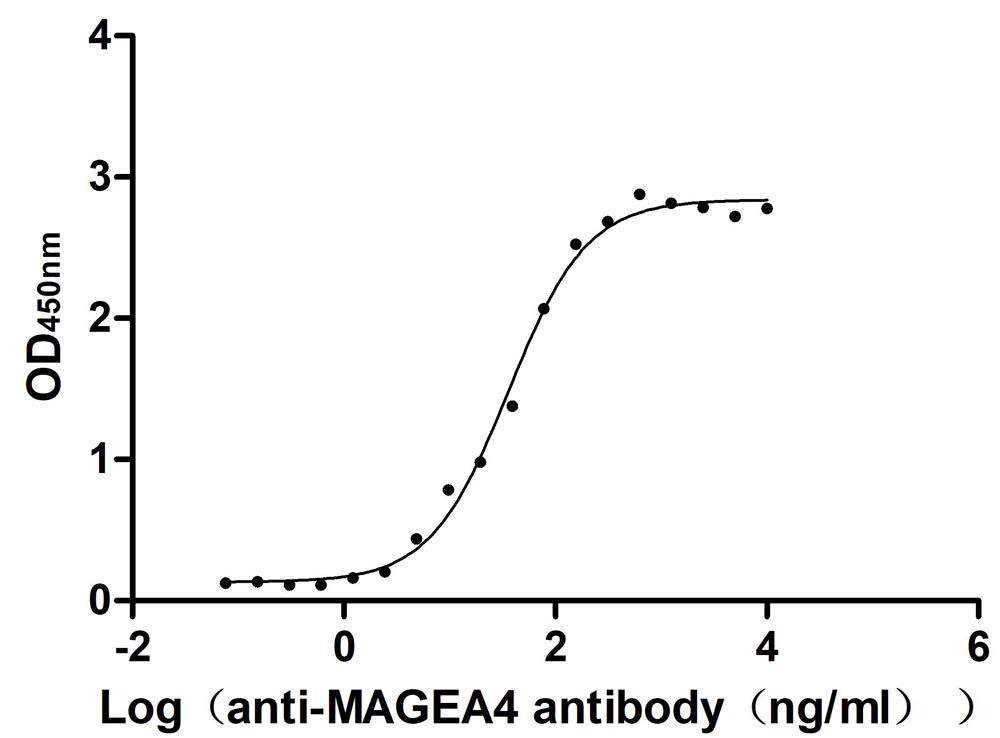
Recombinant Human Melanoma-associated antigen 4 (MAGEA4) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
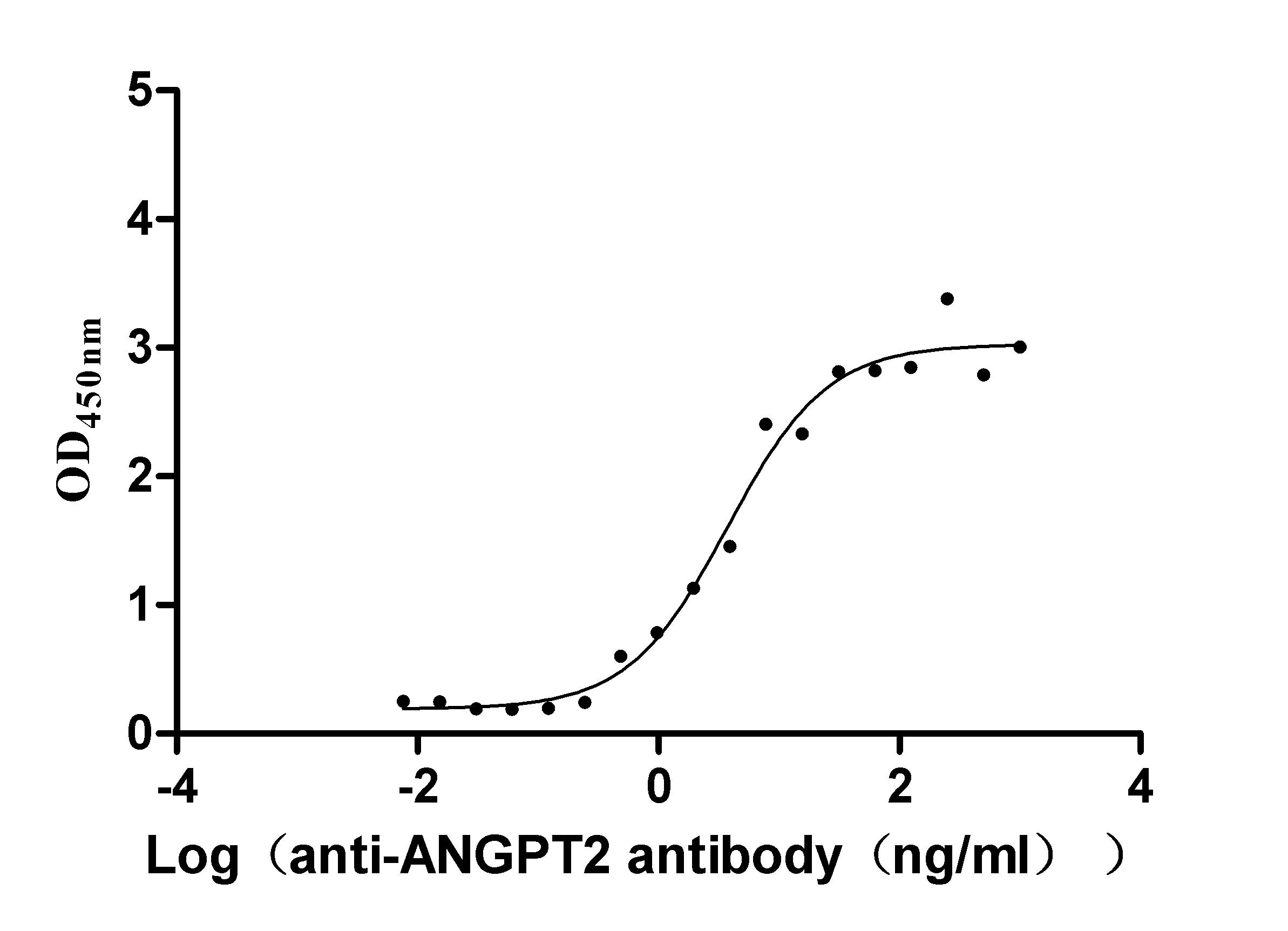
Recombinant Dog Angiopoietin-2 (ANGPT2) (Active)
Express system: Mammalian cell
Species: Canis lupus familiaris (Dog) (Canis familiaris)
-
Recombinant Mouse Complement component C1q receptor (Cd93), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Desmoglein-3 (DSG3), partial (Active)
Express system: Baculovirus
Species: Homo sapiens (Human)
-
Recombinant Human C-type lectin domain family 4 member C (CLEC4C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)