Recombinant Human Small ubiquitin-related modifier 1 (SUMO1)
-
货号:CSB-YP022948HU
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP022948HU
-
规格:
-
来源:E.coli
-
其他:
-
货号:CSB-EP022948HU-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP022948HU
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP022948HU
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:SUMO1
-
Uniprot No.:
-
别名:DAP1; GAP modifying protein 1; GAP-modifying protein 1; GMP 1; GMP1; OFC10; PIC 1; PIC1; SENP2; Sentrin 1; Sentrin; Small ubiquitin related modifier 1; Small ubiquitin-like modifier 1; Small ubiquitin-related modifier 1; SMT3; SMT3 homolog 3; SMT3 suppressor of mif two 3 homolog 1; SMT3, yeast, homolog 3; Smt3C; SMT3H3; SUMO-1; SUMO1; SUMO1_HUMAN; Ubiquitin homology domain protein PIC1; Ubiquitin Like 1; Ubiquitin like protein SMT3C; Ubiquitin like protein UBL1; Ubiquitin-homology domain protein PIC1; Ubiquitin-like protein SMT3C; Ubiquitin-like protein UBL1; UBL 1; UBL1
-
种属:Homo sapiens (Human)
-
蛋白长度:Full Length of Mature Protein
-
表达区域:2-97
-
氨基酸序列SDQEAKPST EDLGDKKEGE YIKLKVIGQD SSEIHFKVKM TTHLKKLKES YCQRQGVPMN SLRFLFEGQR IADNHTPKEL GMEEEDVIEV YQEQTGG
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Ubiquitin-like protein that can be covalently attached to proteins as a monomer or a lysine-linked polymer. Covalent attachment via an isopeptide bond to its substrates requires prior activation by the E1 complex SAE1-SAE2 and linkage to the E2 enzyme UBE2I, and can be promoted by E3 ligases such as PIAS1-4, RANBP2 or CBX4. This post-translational modification on lysine residues of proteins plays a crucial role in a number of cellular processes such as nuclear transport, DNA replication and repair, mitosis and signal transduction. Involved for instance in targeting RANGAP1 to the nuclear pore complex protein RANBP2. Covalently attached to the voltage-gated potassium channel KCNB1; this modulates the gating characteristics of KCNB1. Polymeric SUMO1 chains are also susceptible to polyubiquitination which functions as a signal for proteasomal degradation of modified proteins. May also regulate a network of genes involved in palate development. Covalently attached to ZFHX3.
-
基因功能参考文献:
- SUMO1 expression results in a gain of PKR activity by increasing its activation. SUMO1 was able to activate PKR and eIF-2alpha in the absence of viral infection. PMID: 29352251
- SPOP inhibits hepatocellular carcinoma (HCC) cell metastasis via ubiquitin-dependent SUMO1/SENP7 proteolysis and may thus serve as a new opinion for the prevention of HCC metastasis. PMID: 29777712
- Study found a significant difference in the expression of Cx43 and SUMO1 between cancer stem cells and non-cancer stem cells in liver cancer. By the co-expression of Cx43 and SUMO1 in cancer stem cells, the gap junction intercellular communication of liver cancer stem cells was obviously improved. PMID: 29393359
- Two SUMO modification sites existed in dopamine receptor D1, the phosphorylation of which, due to SUMO modification, can interact with PP2A, leading to the inhibition of D1 de-phosphorylation and normal function. PMID: 28770955
- These results suggest that SUMO1 contributes to hepatocellular carcinoma progression by promoting p65 nuclear translocation PMID: 26993772
- In summary, our study revealed a negative regulation of the UPR transducer ATF6 through post-translational SUMOylation. The information from this study will not only increase our understanding of the fine-tuning regulation of the UPR signaling but will also be informative to the modulation of the UPR for therapeutic benefits. PMID: 29061306
- Molecular dynamics simulations showed that binding of the beta-grasp domain of SUMO1 induces significant conformational and dynamic changes in SENP1, including widening of the exosite cleft and quenching of nanosecond dynamics in all but a distal region. PMID: 27576863
- Mutational analysis of functional sites showed that both peroxidase and PLA2 active sites were necessary for mutant Prdx6 function, and that Prdx6 phosphorylation (at T177 residue) was essential for optimum PLA2 activity.Mutant Prdx6 at its Sumo1 sites escapes and abates this adverse process by maintaining its integrity and gaining function PMID: 28055018
- SUMO and p21Cip1 regulate the transit of proteins through the nucleolus; disruption of nucleolar export by DNA damage induces SUMO and p21Cip1 to act as hub proteins to form a multiprotein complex in the nucleolus. PMID: 28582471
- This study reveals an essential role of SUMOylated FADD in Drp1- and caspase-10-dependent necrosis. PMID: 27799292
- SUMO-1 gene silencing inhibits proliferation and promotes apoptosis of human gastric cancer cells. PMID: 28222440
- the critical role of Cys52 in maintaining SUMO-1 conformation and function PMID: 27195426
- Findings suggest SUMO-1 protein and PGE2 receptor subtype 4 (EP4) as two potential targets for new therapeutic or prevention strategies for endometrial cancers. PMID: 27230680
- This study demonstrated that the rs12472035 polymorphism of SUMO1 was significantly associated with an increased risk of AD in male group. PMID: 27084229
- FOXP2 can be modified with all three human SUMO proteins and that PIAS1 promotes this process. PMID: 26867680
- Ang II-induced upregulation of ATF3 and SUMO1 in vitro and in vivo was blocked by Ang II type I receptor antagonist olmesartan. Moreover, Ang II induced ATF3 SUMOylation at lysine 42, which is SUMO1 dependent. PMID: 26850942
- Data show that mutation of key residues in the binding site abolishes binding and that small ubiquitin-like modifier 1 (SUMO1) can simultaneously and non-cooperatively bind both the ZZ domain and a canonical SIM motif of CREB-binding protein (CBP/p300). PMID: 27129204
- Roles for SUMO in pre-mRNA processing PMID: 26563097
- SUMOylation at specific sites on PXR protein are involved in enhancement of transcription function of this receptor. PMID: 26549688
- High DAP1 expression is associated with a 4-fold increase in the risk of lymph node metastases in squamous cell carcinoma of the oral cavity. PMID: 26400283
- Knockdown of SUMO1 using specific siRNA influenced the accumulation of lipid droplets and reduced HCV replication. PMID: 26449956
- The LKB1 K178R SUMO mutant had defective AMPK signaling and mitochondrial function, inducing death in energy-deprived cells. PMID: 26212320
- Data suggest that small ubiquitin-related modifier protein SUMO1 modification of the promyelocytic leukemia protein (PML) RING domain promotes SUMO2 conjugation to Lys160. PMID: 26060329
- Data identify PDGFRbeta as the hub gene in both inflammatory (IBC) and non-inflammatory breast neoplasm (non-IBC) and SUMO1 and COL1A1 the respective key genes for IBC and non-IBC suggesting they might play important role in the pathogenesis of the neoplasm. PMID: 26403314
- PML IV/ARF interaction enhances p53 SUMO-1 conjugation, activation, and senescence. PMID: 26578773
- Data indicate that small ubiquitin-like modifiers SUMO1, SUMO2, or SUMO3 were found in nuclear speckles. PMID: 26223657
- These results confirm that the SUMO machinery is involved in TRIM5alpha-mediated retroviral restriction, and demonstrate that TRIM5alpha is a SUMO 1 and SUMO 2 substrate. PMID: 25880753
- Under cell-free in vitro conditions, p35 is covalently modified by SUMO1. Sumoylation is a likely candidate mechanism for the rapid modulation of p35/Cdk5 activity in physiological situations as well as in disease. PMID: 25391294
- SUMO1 modification stabilizes CDK6 protein and drives the cell cycle and glioblastoma progression. PMID: 24953629
- SUMO1 accelerates the accumulation of autophagic vacuoles and promotes Abeta production. PMID: 25484073
- Expression of SUMO1/2/3 is dramatically enhanced by interferons through an miRNA-based mechanism involving the Lin28/let-7. PMID: 24942926
- SUMO-1 modification may affect the transcriptional activity of EGFR PMID: 26244656
- The crystal structures of SUMO1 bound to unphosphorylated and tetraphosphorylated PML-SUMO-interacting motifs peptides indicate that three phosphoserines directly contact specific positively charged residues of SUMO1. PMID: 25497731
- The expression of SUMO-1 in OLP was similar to normal mucosa and inflammatory fibrous hyperplasia, suggesting that alterations of this protein occur at later stages of carcinogenesis PMID: 23350884
- reveals an unexpected role of SUMO-1 and SAFB in the stimulatory coupling of promoter binding, transcription initiation and RNA processing PMID: 25800734
- MDM2 and SUMO-1 proteins are up-regulated in actinic cheilitis and lip cancer. PMID: 25241153
- Identify DRP1, RanGAP1 and topoisomerase IIa as targets of SUMO1 in human ejaculated sperm, giving more insights on the role of SUMO1-ylation in sperm morphology and motility. PMID: 25118297
- findings provide empirical evidence that SUMO1 genetic polymorphisms might be strongly involved in the etiology of NSCL/P, especially for rs12470401 T>C, rs16838917 A>G, rs12470529 A>G, and rs7572505 A>G polymorphisms. PMID: 25111678
- Overexpression of SUMO-1 decreased phosphorylated GSK-3beta at Ser-9. Mutagenesis of tau at K340R or inhibition of tau SUMOylation by ginkgolic acid abolishes the effect of SUMO-1. PMID: 25378699
- The mutation of the sumoylation site (Lys137) of PTBP2 markedly inhibited its modification by SUMO1. PMID: 24286314
- SUMO potentiates the inhibition of protein synthesis induced by PKR in response to dsRNA. PMID: 25074923
- These results show that DAP1 is a key regulator, of the induction of apoptosis and reduction of autophagy by subtilase cytotoxin (SubAB). PMID: 25183729
- identified 295 SUMO1 and 167 SUMO2 sites on endogenous substrates of HeLa cells PMID: 25114211
- Modification of TDG by small ubiquitin-like modifier (SUMO) proteins weakens its binding to abasic DNA. PMID: 24753249
- SUMO-1 modification of RARA is a potent mechanism for balancing proliferation and differentiation by controlling the stability of RARA in cancer cells. PMID: 24819975
- SUMO1P3 was significantly up-regulated in gastric cancer tissues. PMID: 23996296
- SUMO chain-induced dimerization activates RNF4. PMID: 24656128
- The mitosis-dependent dynamic SUMO-1 modification of NuMA might contribute to NuMA-mediated formation and maintenance of mitotic spindle poles during mitosis. PMID: 24309115
- PIASxalpha is a novel SUMO E3 ligase for PTEN, and it positively regulates PTEN protein level in tumor suppression. PMID: 24344134
- SUMO participates in transcriptional repression as both a covalent modification and through non-covalent interactions with E2 and E3 enzymes. PMID: 24174529
显示更多
收起更多
-
相关疾病:Non-syndromic orofacial cleft 10 (OFC10)
-
亚细胞定位:Nucleus membrane. Nucleus speckle. Cytoplasm. Nucleus, PML body. Cell membrane. Nucleus.
-
蛋白家族:Ubiquitin family, SUMO subfamily
-
数据库链接:
HGNC: 12502
OMIM: 601912
KEGG: hsa:7341
STRING: 9606.ENSP00000376076
UniGene: Hs.81424
Most popular with customers
-
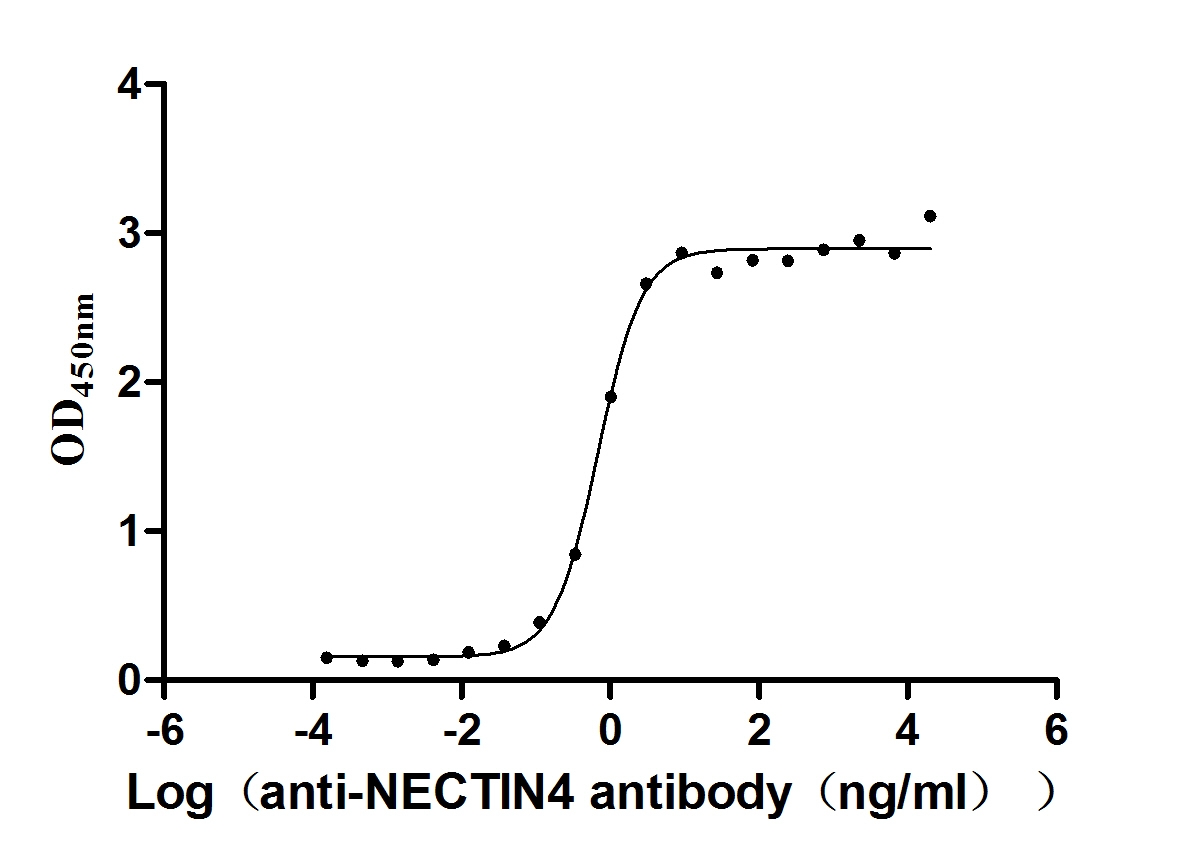
Recombinant Human Nectin-4 (NECTIN4), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human HLA class II histocompatibility antigen gamma chain (CD74), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
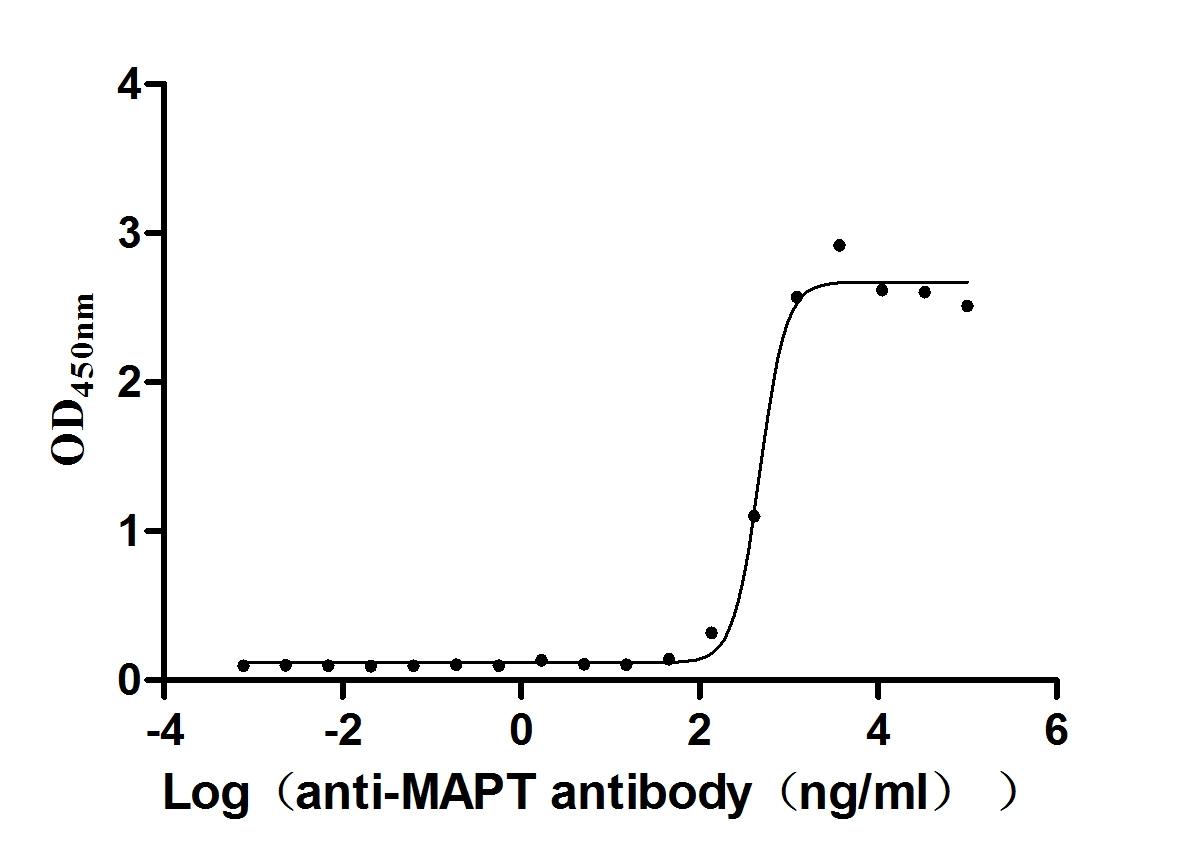
Recombinant Mouse Microtubule-associated protein tau (Mapt) (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
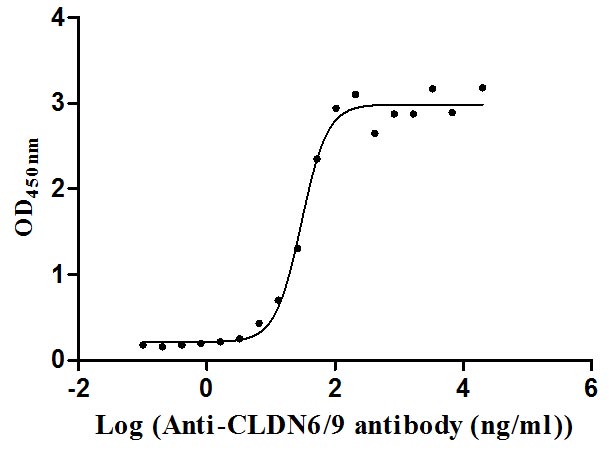
Recombinant Human Claudin-9 (CLDN9)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
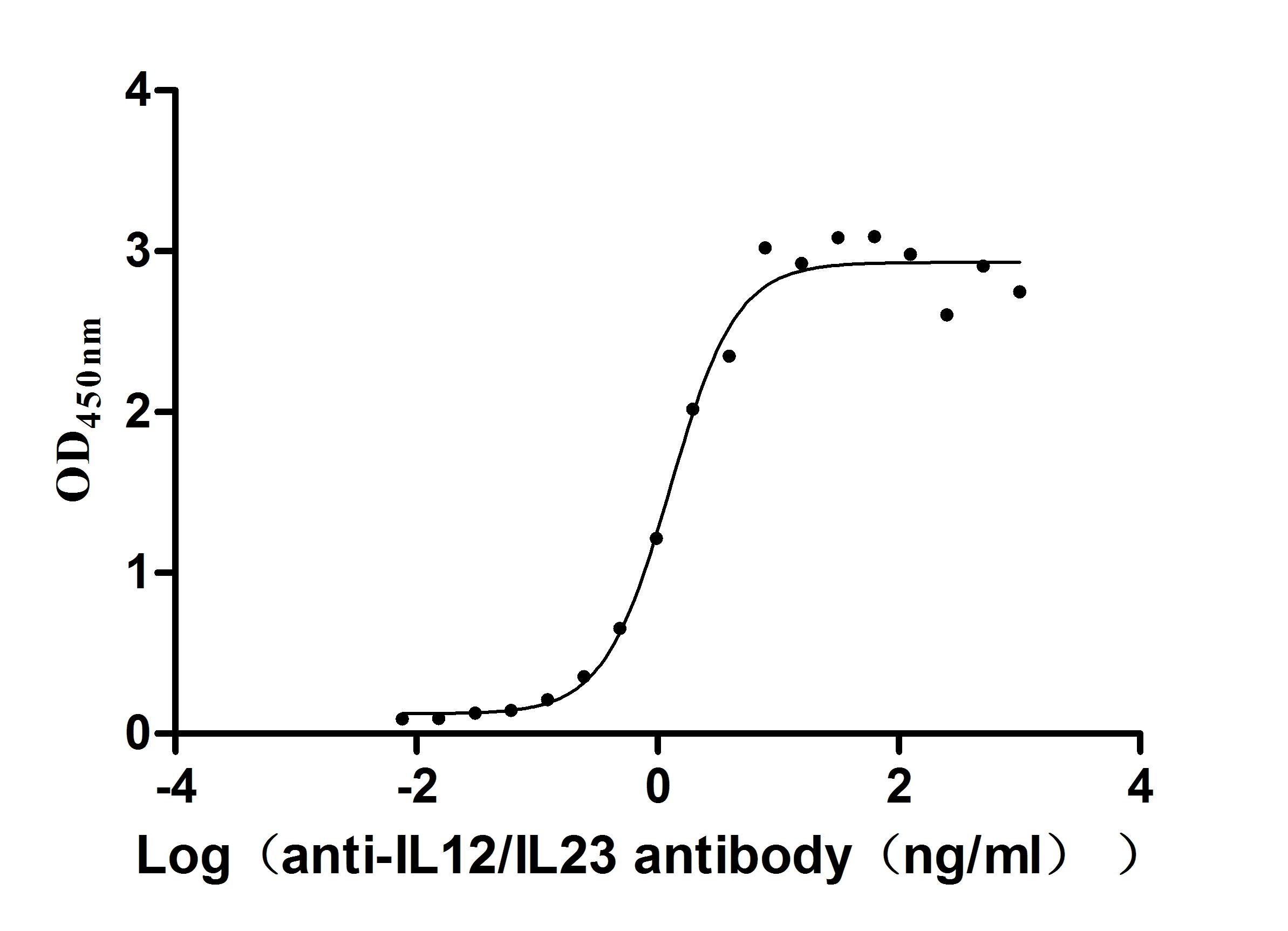
Recombinant Human IL12B&IL12A Heterodimer Protein (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)


-AC1.jpg)