Recombinant Human Speckle-type POZ protein (SPOP)
-
货号:CSB-YP022601HU
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP022601HU
-
规格:
-
来源:E.coli
-
其他:
-
货号:CSB-EP022601HU-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP022601HU
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP022601HU
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
别名:BTBD32; HIB homolog 1; Roadkill homolog 1; Speckle type POZ protein; Speckle-type POZ protein; SPOP; SPOP_HUMAN; TEF 2; TEF2
-
种属:Homo sapiens (Human)
-
蛋白长度:Full length protein
-
表达区域:1-374
-
氨基酸序列MSRVPSPPPP AEMSSGPVAE SWCYTQIKVV KFSYMWTINN FSFCREEMGE VIKSSTFSSG ANDKLKWCLR VNPKGLDEES KDYLSLYLLL VSCPKSEVRA KFKFSILNAK GEETKAMESQ RAYRFVQGKD WGFKKFIRRD FLLDEANGLL PDDKLTLFCE VSVVQDSVNI SGQNTMNMVK VPECRLADEL GGLWENSRFT DCCLCVAGQE FQAHKAILAA RSPVFSAMFE HEMEESKKNR VEINDVEPEV FKEMMCFIYT GKAPNLDKMA DDLLAAADKY ALERLKVMCE DALCSNLSVE NAAEILILAD LHSADQLKTQ AVDFINYHAS DVLETSGWKS MVVSHPHLVA EAYRSLASAQ CPFLGPPRKR LKQS
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Component of a cullin-RING-based BCR (BTB-CUL3-RBX1) E3 ubiquitin-protein ligase complex that mediates the ubiquitination of target proteins, leading most often to their proteasomal degradation. In complex with CUL3, involved in ubiquitination and proteasomal degradation of BRMS1, DAXX, PDX1/IPF1, GLI2 and GLI3. In complex with CUL3, involved in ubiquitination of MACROH2A1 and BMI1; this does not lead to their proteasomal degradation. Inhibits transcriptional activation of PDX1/IPF1 targets, such as insulin, by promoting PDX1/IPF1 degradation. The cullin-RING-based BCR (BTB-CUL3-RBX1) E3 ubiquitin-protein ligase complex containing homodimeric SPOP has higher ubiquitin ligase activity than the complex that contains the heterodimer formed by SPOP and SPOPL. Involved in the regulation of bromodomain and extra-terminal motif (BET) proteins BRD2, BRD3, BRD4 stability.
-
基因功能参考文献:
- SPOP inhibits hepatocellular carcinoma (HCC) cell metastasis via ubiquitin-dependent SENP7 proteolysis and may thus serve as a new opinion for the prevention of HCC metastasis. PMID: 29777712
- Our result suggests that SPOP expression in blood might have a sensitivity that is low for routine diagnostic use and for screening for Renal cell carcinoma (RCC). However, SPOP could be a potential tissue diagnostic biomarker in RCC. PMID: 30197334
- Expression of the F133V mutation and wild-type SPOP was at much lower levels compared to that of F102C and Y87N mutations; however, at present, it is unknown if this also affects the biological activity of the SPOP protein. PMID: 28810879
- PD-L1 protein abundance is regulated by cyclin D-CDK4 and the cullin 3-SPOP E3 ligase via proteasome-mediated degradation PMID: 29160310
- methylation status of SPOP promoter can be used as a novel epigenetic biomarker and a therapeutic target in colorectal cancer. PMID: 28032859
- these data highlight SPOP as an important regulator of luminal epithelial cell proliferation and c-MYC expression in prostate physiology, identify c-MYC as a novel bona fide SPOP substrate, and help explain the frequent inactivation of SPOP in human prostate adenocarcinoma. PMID: 28414305
- Results elucidate the tumor-suppressor role of SPOP in prostate cancer in which it acts as a negative regulator of BET protein stability and also provide a molecular mechanism for resistance to BET inhibitors in individuals with prostate cancer bearing SPOP mutations. PMID: 28805820
- SPOP mutation in endometrial cancer increased degradation of BRD2, BRD3 and BRD4 proteins. SPOP mutation in prostate cancer increased expression of BRD2, BRD3 and BRD4 proteins. PMID: 28805821
- Prostate cancer-derived SPOP mutants failed to interact with Cdc20 to promote its degradation. As a result, SPOP-deficient prostate cancer cells with elevated Cdc20 expression became resistant to a pharmacological Cdc20 inhibitor. PMID: 27780719
- While wild-type SPOP localizes to liquid nuclear speckles, self-association-deficient SPOP mutants have a diffuse distribution in the nucleus. SPOP oligomerizes through its BTB and BACK domains. PMID: 27220849
- SPOP mutation activates both PI3K/mTOR and androgen receptor signaling, effectively uncoupling the normal negative feedback between these two pathways. PMID: 28292441
- The levels of SPOP significantly decreased, while the levels of SIRT2 significantly increased in non-small cell lung cancer (NSCLC) cell lines, compared to normal bronchial epithelial cell line and NSCLC specimens, compared to the paired non-tumor lung tissue. PMID: 28073696
- hese findings reveal novel molecular events underlying the regulation of INF2 function and localization, and provided insights in understanding the relationship between SPOP mutations and dysregulation of mitochondrial dynamics in prostate cancer. PMID: 28448495
- Results show that SPOP is highly expressed in clear cell renal cell carcinoma (RCC) and associated with disease progression. In vitro, SPOP promotes the invasiveness of RCC. PMID: 27572476
- Data indicate that a mutation in the SPOP gene may not be associated with breast cancer, particularly in Chinese women. PMID: 26505385
- SPOP acts as a tumor suppressor by promoting senescence through degrading SENP7. PMID: 26527005
- our findings emphasize the critical role of SPOP in the regulation of proliferation and migration in liver cancer PMID: 26156804
- results suggest that SPOP plays a pivotal role in colorectal cancer (CRC) through mesenchymal-epithelial transition and matrix metalloproteases. PMID: 26022775
- SPOP functions as a tumor suppressor to negatively regulate the stability of the ERG oncoprotein in prostate cancer. PMID: 26344095
- Overcoming ERG resistance to SPOP-mediated degradation represents a viable strategy for treatment of prostate cancers expressing either mutated SPOP or truncated ERG. PMID: 26344096
- Our study revealed novel molecular mechanisms underlying the regulation of ERa protein and provided insights in understanding the relationship between SPOP mutations and the development of endometrial cancer. PMID: 25766326
- SPOP plays critical roles in suppressing gastric tumorigenesis through inhibiting Hh/Gli2 signaling pathway. It may provide an alternative strategy for developing therapeutic agents of gastric cancer in future. PMID: 25204354
- SPOP mutations and novel variants were detected in 5 of 27 aggressive PCa and one of 22 less aggressive PCa PMID: 24994784
- SPOP has potential use as novel biomarker of glioma and may serve as an independent predictive factor for prognosis of glioma patients. PMID: 25351530
- This study reveals novel molecular events underlying the regulation of DDIT3 protein homeostasis and provides insight in understanding the relationship between SPOP mutations and ER stress dysregulation in prostate cancer. PMID: 24990631
- SPOP mutations contribute to prostate cancer development by altering the steady-state levels of key components in the androgen-signaling pathway; mutations are also observed in endometrial cancers PMID: 25058385
- studies highlight the AR axis as the key transcriptional output of SPOP in prostate adenocarcinoma and provide an explanation for the prostate-specific tumor suppressor role of wt-SPOP PMID: 25274033
- The present study demonstrates that prognosis varies depending on SPOP expression and mutational status, hence, defining a new biotype of PCa associated with a worse prognosis. PMID: 25204806
- miR-145 has a role in post-transcriptional regulation of SPOP expression in selected tissues. PMID: 24845504
- Prostate-cancer-associated SPOP mutants cannot bind to and promote androgen receptor degradation. PMID: 24508459
- SPOP mutants impaired ubiquitylation of a subset of proteins in a dominant-negative fashion. PMID: 25278611
- a novel mutation in SPOP that tracks with prostate cancer within a family and is predicted to be deleterious. Taken together, our results implicate SPOP as a candidate gene for hereditary prostate cancer PMID: 24796539
- the Speckle-Type POZ Protein (SPOP) gene is mutated in prostate cancer in a manner that is not specific for ethnicity, clinical, or pathologic parameters PMID: 24563616
- Results indicate that SPOP serves as a regulatory hub to promote ccRCC tumorigenesis. PMID: 24656772
- Prevalence of SPOP gene mutation varies depending on cancer types. It is common in prostate cancer and endometrial cancer, and rare in breast cancer, lung cancer, liver cancer, and acute leukemias. PMID: 23654205
- Crystal structure of the high-order SPOP oligomer is presented depicting a helical organization that could enhance the efficiency of substrate ubiquitination. PMID: 23999291
- Dzip1-dependent stabilization of Spop/HIB is evolutionarily conserved and essential for proper regulation of Gli/Ci proteins in the Hh pathway. PMID: 24072710
- Loss of expression of SPOP gene might play a role in cancer pathogenesis by altering tumor suppressor gene functions of SPOP. PMID: 23216165
- SPOP plays a critical tumor suppressor role in prostate cancer cells, promoting the turnover of SRC-3 protein and suppressing androgen receptor transcriptional activity. PMID: 23559371
- Adaptor protein self-assembly provides a graded level of regulation of the SPOP/Cul3 E3 ligase toward its multiple protein substrates. PMID: 22632832
- These results suggest that the novel regulatory mechanism of BRMS1 by Cul3-SPOP complex is important for breast cancer progression. PMID: 22085717
- Mutagenesis study suggests that the ability of SPOP to self-associate as well as its ability to bind with Daxx was important for the modulation of Daxx-mediated transcriptional repression. PMID: 15240113
- SPOP/Cul3-ubiquitin ligase plays an essential role in the control of Daxx level and, thus, in the regulation of Daxx-mediated cellular processes, including transcriptional regulation and apoptosis PMID: 16524876
- define a novel mechanism whereby the phosphoinositide phosphatidylinositol 5-phosphate leads to stimulation of Cul3-SPOP ubiquitin ligase activity PMID: 18218622
- The study first proposes the proapoptotic aspect of the BTB/POZ domain of SPOP protein based on the finding that cells expressing the C-terminal fragment of SPOP containing the BTB/POZ domain underwent apoptosis. PMID: 18997279
- study found that SPOP plays a conserved role in TNF-mediated JNK signaling and was highly expressed in 99% of clear cell renal cell carcinomas PMID: 19164706
- The study provides a molecular understanding of how SPOP and other MATH-BTB proteins recruit substrates to Cul3 and how their dimerization and conformational variability may facilitate avid interactions with diverse substrates. PMID: 19818708
- Here, we report that the E3 ubiquitin ligase consisting of SPOP and CULLIN3 is able to ubiquitinate the Polycomb group protein BMI1. PMID: 15897469
显示更多
收起更多
-
亚细胞定位:Nucleus. Nucleus speckle.
-
蛋白家族:Tdpoz family
-
组织特异性:Widely expressed.
-
数据库链接:
HGNC: 11254
OMIM: 602650
KEGG: hsa:8405
STRING: 9606.ENSP00000240327
UniGene: Hs.463382
Most popular with customers
-
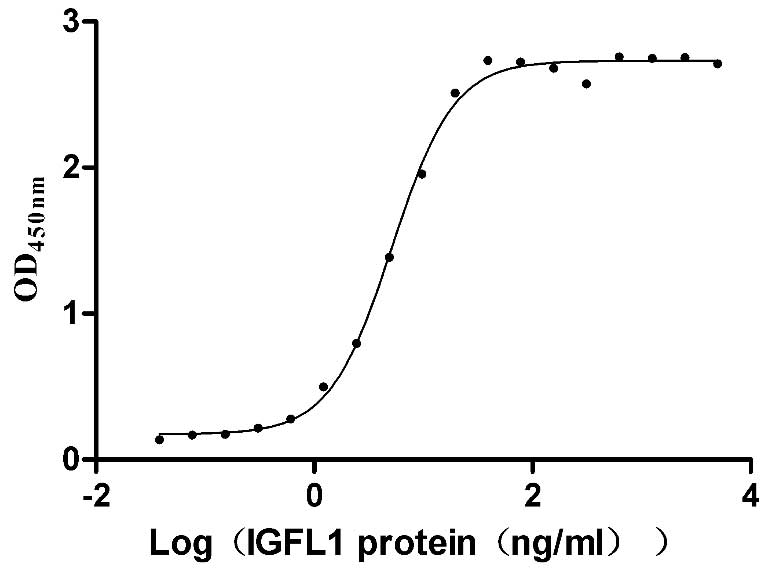
Recombinant Human IGF-like family receptor 1 (IGFLR1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
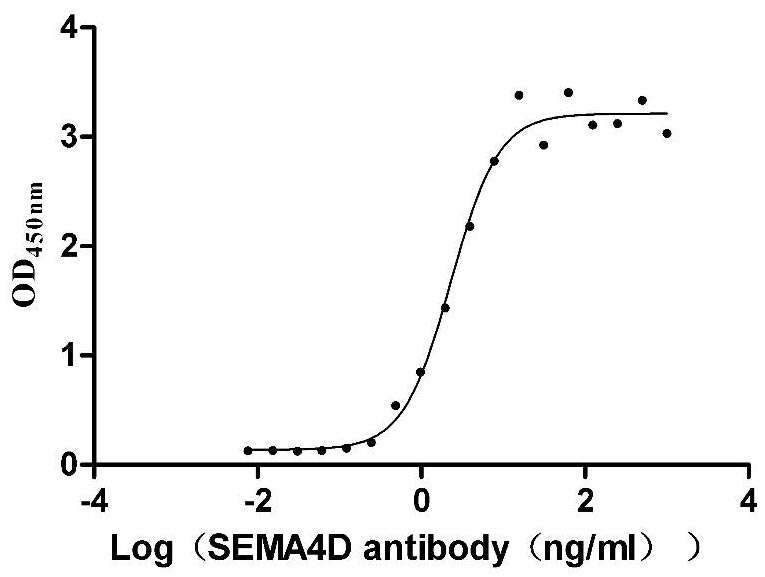
Recombinant Macaca mulatta Semaphorin-4D isoform 1 (SEMA4D), partial (Active)
Express system: Mammalian cell
Species: Macaca mulatta (Rhesus macaque)
-
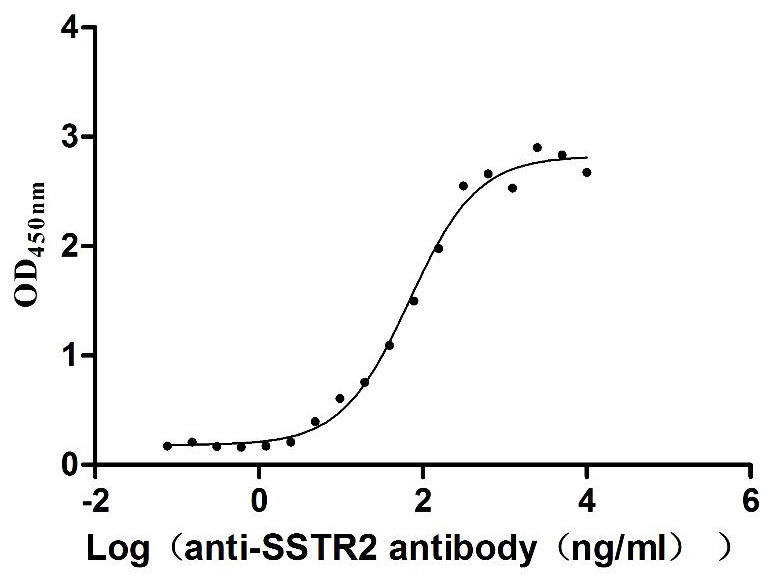
Recombinant Human Somatostatin receptor type 2 (SSTR2)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
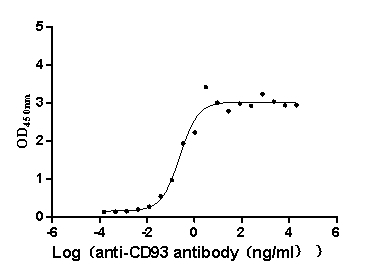
Recombinant Macaca fascicularis CD93 molecule (CD93), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
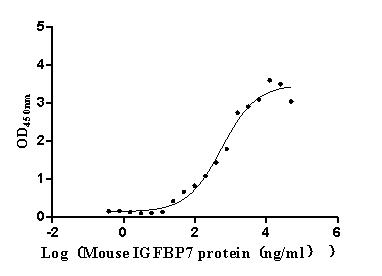
Recombinant Mouse Complement component C1q receptor (Cd93), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Human Claudin-6 (CLDN6)-VLPs, Fluorescent (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
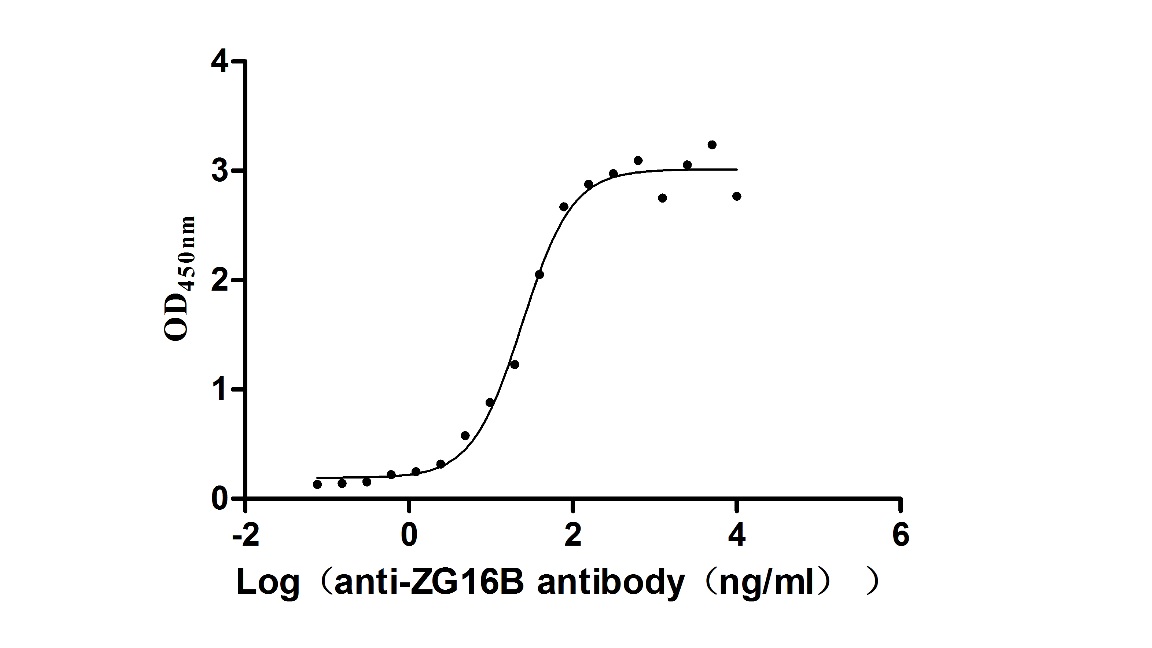
Recombinant Macaca fascicularis zymogen granule protein 16 homolog B (ZG16B) (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
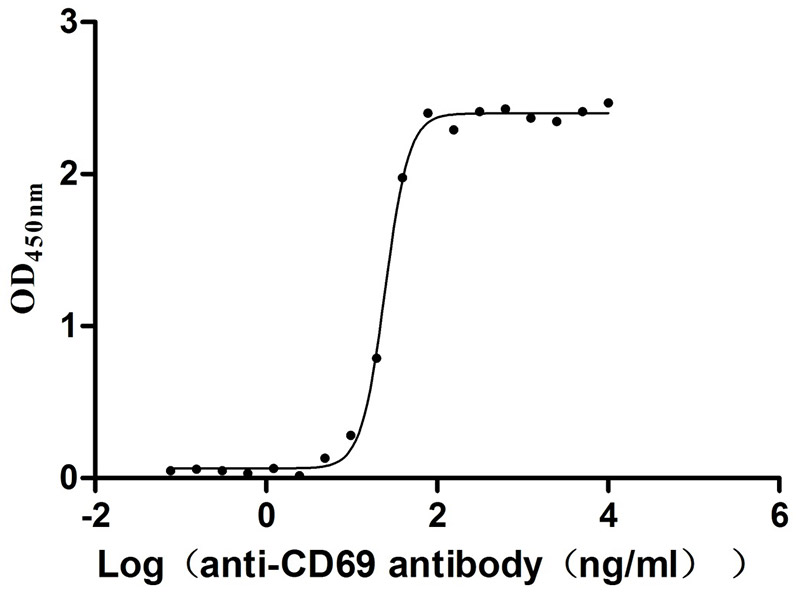
Recombinant Human Early activation antigen CD69 (CD69), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)


f4-AC1.jpg)