乳腺癌
乳腺癌是一种起源于乳腺上皮细胞的恶性肿瘤,是女性中最常见的癌症之一,也是导致女性癌症死亡的主要原因之一。乳腺癌的发病率与多种因素有关,包括遗传、年龄、激素水平、生活方式等。根据肿瘤细胞的特征,乳腺癌可以分为不同的亚型,如激素受体阳性(ER+/PR+)、人表皮生长因子受体2(HER2+)和三阴性乳腺癌(TNBC)等。
近年来,乳腺癌的治疗和药物研发取得了显著进展。特别是在靶向治疗和免疫治疗领域,新药物和新疗法的不断涌现为患者带来了更多的治疗选择和更好的预后。
靶向治疗药物
● HER2阳性乳腺癌
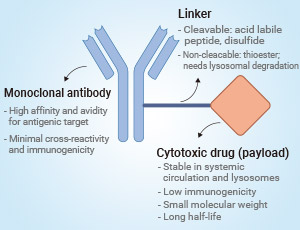
HER2阳性乳腺癌的治疗在过去几年中取得了重大进展。ADC(抗体药物偶联物)类药物治疗是HER2阳性乳腺癌治疗的重要突破。例如,DS-8201(Trastuzumab Deruxtecan)是一种针对HER2的ADC药物,已经在临床试验中显示出对HER2低表达和HER2阴性乳腺癌患者的潜在疗效67。此外,T-DM1(Trastuzumab Emtansine)和T-DXd(Trastuzumab Deruxtecan)也在HER2阳性乳腺癌的治疗中取得了成功。
● 激素受体阳性乳腺癌
对于激素受体阳性的乳腺癌,CDK4/6抑制剂如阿贝西利(Abemaciclib)、达尔西利(Dalpiciclib)和瑞波西利(Ribociclib)等药物的开发为患者提供了新的治疗选择。这些药物通过抑制细胞周期的关键调节因子,减缓肿瘤细胞的生长和扩散。
● 三阴性乳腺癌
三阴性乳腺癌(TNBC)是一种较为难治的亚型,因为它缺乏激素受体和HER2的表达。然而,免疫治疗在这一领域取得了进展,例如PD-1/PD-L1抑制剂在TNBC的治疗中显示出潜力。
免疫治疗药物
免疫治疗是乳腺癌治疗领域的另一个重要进展。免疫检查点抑制剂,如PD-1和PD-L1抑制剂,已经在三阴性乳腺癌的治疗中显示出一定的疗效。此外,研究者正在探索将免疫治疗与其他治疗方法结合使用的策略,以提高治疗效果。
乳腺癌药物靶点
- CA9
- CD247
- CD27
- CD274
- CD276
- CD28
- CD40
- CD44
- CD47
- CEACAM5
- CSF1R
- CSF2
- CSF3R
- CTAG1A
- CTLA4
- DNA2
- EGFR
- EpCAM
- EpoR
- ERBB2
- ERBB3
- FCGR1A
- FCY1
- FGFR3
- FOLR1
- HMMR
- ICAM1
- ICOS
- IGF1
- IGF1R
- IGF2
- IL12A
- IL12RB1
- IL15RA
- IL2
- IL2RA
- IL2RB
- KDR
- LAG3
- MELTF
- MET
- MSLN
- MUC1
- MUC16
- NECTIN4
- NMT1
- NMT2
- NT5E
- PDCD1
- PRLR
- PTK7
- PVRIG
- ROR1
- SIRPA
- STING1
- TACSTD2
- TF
- TGFB1
- TGFB2
- TIGIT
- TLR8
- TNFRSF10B
- TNFRSF1A
- TNFRSF4
- TNFSF11
- TNFSF9
- TOP1
- TPBG
- VEGFA
- VSIR
- VTCN1
乳腺癌药物靶点相关产品推荐
● 靶点蛋白
CSB-MP007763HU
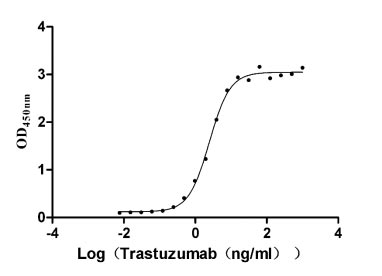
Measured by its binding ability in a functional ELISA. Immobilized HER2 at 2 μg/ml can bind Trastuzumab, the EC50 is 2.179-2.825 ng/ml.
CSB-MP619964HU1
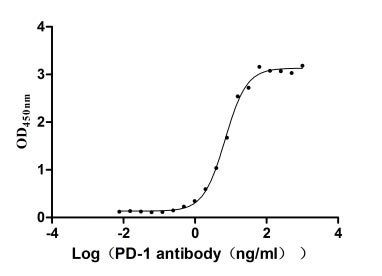
Measured by its binding ability in a functional ELISA. Immobilized PD-1 at 2 μg/ml can bind Anti-PD-1 recombinant antibody, the EC50 of human PD-1 protein is 6.087-7.854 ng/ml.
CSB-MP878942HU1
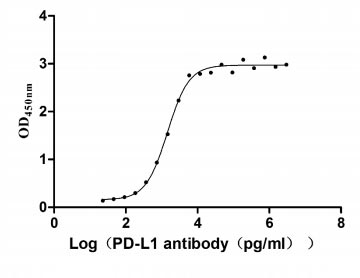
Measured by its binding ability in a functional ELISA. Immobilized PD-L1 at 2 μg/ml can bind Anti- PD-L1 mouse monoclonal antibody (CSB-MA878942A1m, antigen from E.coli), the EC50 of human PD-L1 protein is 1.252-1.653 ng/mL
CSB-MP023072HU1
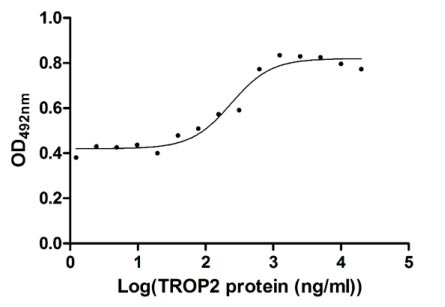
Measured in cell activity assay using U937 cells, the EC50 for this effect is 190.2-298.6 ng/ml.
CSB-MP007765HU
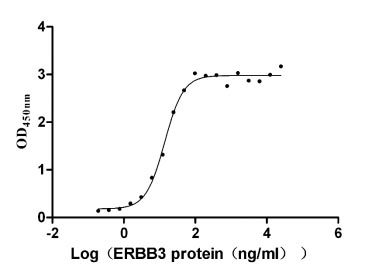
Measured by its binding ability in a functional ELISA. Immobilized NRG1 (CSB-MP016077HU1(F6)) at 2 μg/ml can bind human ERBB3, the EC50 is 12.32-15.74 ng/ml.
CSB-MP006163HU1
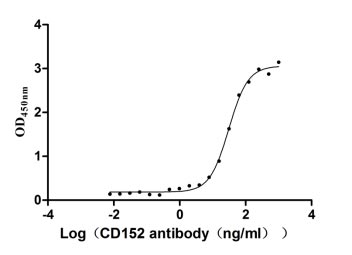
Measured by its binding ability in a functional ELISA. Immobilized CD152 at 2 μg/ml can bind Anti-CD152 rabbit monoclonal antibody (CSB-RA213310A0HU), the EC50 of human CD152 protein is 27.14-34.82 ng/ml.
CSB-MP004940HU
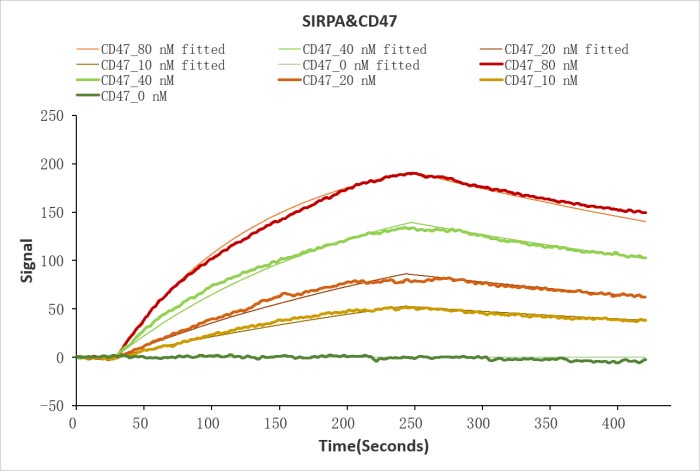
Human SIRPA protein His/Myc tag (CSB-MP021334HU) captured on COOH chip can bind Human CD47 protein Fc tag (CSB-MP004940HU) with an affinity constant of 19.1 nM as detected by LSPR Assay.
CSB-MP007479HU
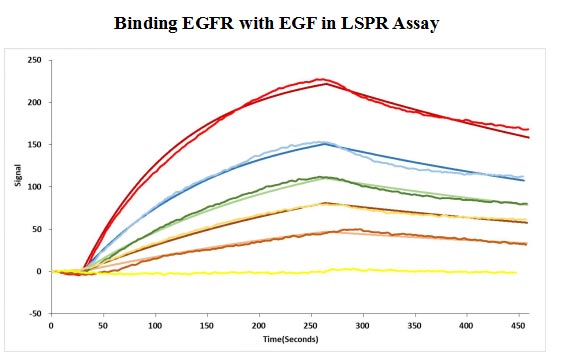
Human EGF protein captured on COOH chip can bind Human EGFR protein, his and Myc tag (CSB-MP007479HU) with an affinity constant of 11.9nM as detected by LSPR Assay.
● 稳定细胞株
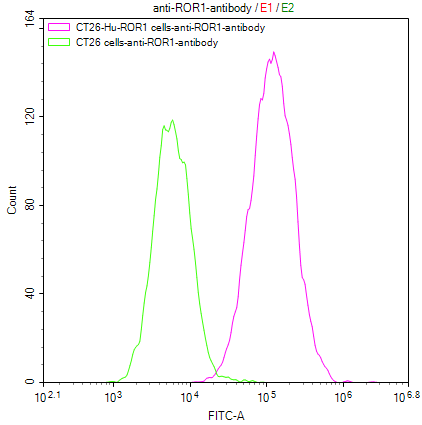
CT26/Human ROR1 Stable Cell Line
CSB-SC020067HU
Untransfected CT26 cells (green line) and transfected Human ROR1 CT26 stable cells (red line) were stained with anti-ROR1 antibody (CSB-RA020067A1HU) (2µg/1*106 cells), washed and then followed by FITC-conjugated anti-Human IgG Fc antibody and analyzed with flow cytometry.
● 重组抗体
CSB-RA260392A0HU
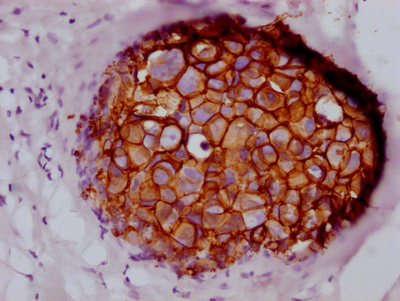
IHC image of CSB-RA260392A0HU diluted at 1:100 and staining in paraffin-embedded human breast cancer performed on a Leica BondTM system.
CSB-RA634199A0HU
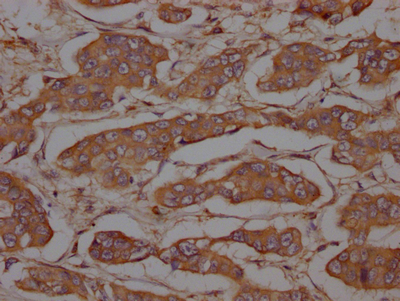
IHC image of CSB-RA634199A0HU diluted at 1:100 and staining in paraffin-embedded human breast cancer performed on a Leica BondTM system.
CSB-RA292372A0HU
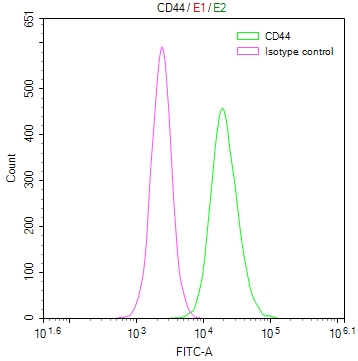
Overlay Peak curve showing Hela cells surface stained with CSB-RA292372A0HU (red line) at 1:50.
CSB-RA159341A0HU
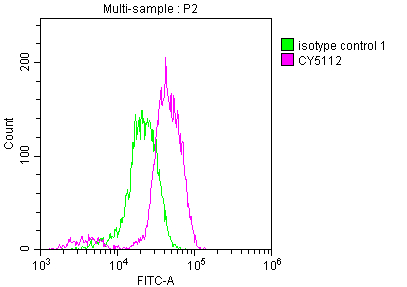
Overlay histogram showing Jurkat cells stained with CSB-RA159341A0HU (red line) at 1:50.
| 产品名称 | 货号 | 靶点 | 反应种属 | 应用范围 |
|---|---|---|---|---|
| CA9 Recombinant Monoclonal Antibody | CSB-RA614990A0HU | CA9 | Human | ELISA, IHC |
| CD247 Recombinant Monoclonal Antibody | CSB-RA244537A0HU | CD247 | Human | ELISA, FC |
| CD27 Recombinant Monoclonal Antibody | CSB-RA953976A0HU | CD27 | Human | ELISA, WB |
| CD274 Recombinant Monoclonal Antibody | CSB-RA977797A0HU | CD274 | Human | ELISA, WB, IHC |
| CD274 Recombinant Monoclonal Antibody | CSB-RA878942MA1HU | CD274 | Human | ELISA, IHC, FC |
| CD40 Recombinant Monoclonal Antibody | CSB-RA004936MA1HU | CD40 | Human | ELISA, IF, FC |
| CD44 Recombinant Monoclonal Antibody | CSB-RA004938A0HU | CD44 | Human | ELISA, WB, IHC |
| CD44 Recombinant Monoclonal Antibody | CSB-RA292372A0HU | CD44 | Human | ELISA, WB, IHC, IF, FC |
| CD44 Recombinant Monoclonal Antibody | CSB-RA004938MA1HU | CD44 | Human | ELISA, FC |
| CD47 Recombinant Monoclonal Antibody | CSB-RA802124A0HU | CD47 | Human | ELISA, WB, IHC, IF |
| CEACAM5 Recombinant Monoclonal Antibody | CSB-RA005165MA3HU | CEACAM5 | Human | ELISA, IF, FC |
| CEACAM5 Recombinant Monoclonal Antibody | CSB-RA005165MA1HU | CEACAM5 | Human | ELISA |
| CTLA4 Recombinant Monoclonal Antibody | CSB-RA213310A0HU | CTLA4 | Human | ELISA, IHC |
| CTLA4 Recombinant Monoclonal Antibody | CSB-RA006163MA1HU | CTLA4 | Human, Mouse | ELISA, WB, IF, FC |
| Phospho-EGFR (Y1092) Recombinant Monoclonal Antibody | CSB-RA007479A1092phHU | EGFR | Human | ELISA, WB |
| Phospho-EGFR (Y1068) Recombinant Monoclonal Antibody | CSB-RA007479A1068phHU | EGFR | Human | ELISA, WB |
| EGFR Recombinant Monoclonal Antibody | CSB-RA159341A0HU | EGFR | Human | ELISA, WB, IHC, IF, FC |
| EGFR Recombinant Monoclonal Antibody | CSB-RA794061A0HU | EGFR | Human | ELISA, WB, IHC |
| EGFR Recombinant Monoclonal Antibody | CSB-RA159341MA1HU | EGFR | Human | ELISA, IHC, FC |
| EPCAM Recombinant Monoclonal Antibody | CSB-RA932207A0HU | EPCAM | Human | ELISA, WB |
● ELISA试剂盒
药物研发解决方案
专家讲堂
参考文献:
1. Cell 186, April 13, 2023
2. Larissa A. Korde, Mark R. Somerfield, Dawn L. Hershman, et al. Use of Immune Checkpoint Inhibitor Pembrolizumab in the Treatment of High-Risk, Early-Stage Triple-Negative Breast Cancer: ASCO Guideline Rapid Recommendation Update. DOI: 10.1200/JCO.22.00503 Journal of Clinical Oncology
3. Lajos Pusztai, Carsten Denkert, Joyce O'Shaughnessy, et al. Event-free survival by residual cancer burden after neoadjuvant pembrolizumab + chemotherapy versus placebo + chemotherapy for early TNBC: Exploratory analysis from KEYNOTE-522. J Clin Oncol 40, 2022 (suppl 16; abstr 503). DOI: 10.1200/JCO.2022.40.16_suppl.503
4. Breast cancer-WHO: https://www.who.int/news-room/fact-sheets/detail/breast-cancer